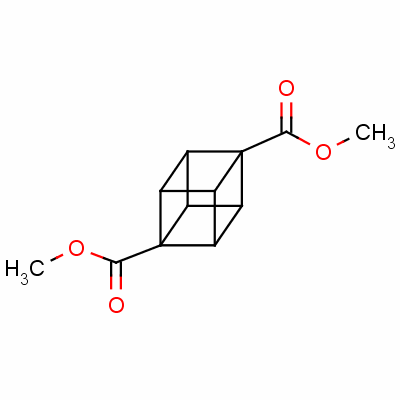
Dimethyl cubane-1,4-dicarboxylate
dimethyl 1,4-cubanedicarboxylate;
1,4-cubanedicarboxylic acid dimethyl*ester;
methyl 4-(methoxycarbonyl)pentacyclo[4.2.0.0<2,5>.0<3,8>.0<4,7>]octanecarboxylate
Pentacyclo(4.2.0.0(2,5).0(3,8).0(4,7))octane-1,4-dicarboxylic acid dimethyl ester
CAS 29412-62-2
| Molecular Weight: | 220.2213 |
| Molecular Formula: | C12H12O4 |
| Density: | 1.684g/cm3 |
| Boiling Point(℃): | 270°C at 760 mmHg |
| Flash Point(℃): | 131.3°C |
| refractive_index: | 1.704 |

An interesting OPRD paper on the scale up of dimethyl cubane -1,4-dicarboxylate.
The work appeared in Organic Process Research and development, 2013, doi.org/10.1021/op400181g . It was carried out by an Australian group, John Tsanaktsidis, Michael Falkiner, Stuart Littler, Kenneth McRae and Paul Savage from CSIROand features a large-scale photochemical reaction which is very unusual to see in a scaled chemical process.
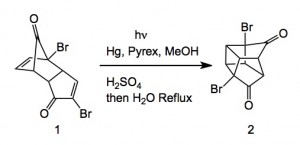
Extending their previous work from 1997, they scaled the following reaction.
As is the norm with such reactions the reaction requires high dilution to be successful. In this case they used a tailor made photochemical reactor. A solution of 1 in methanol/water was pumped through the reactor at 4 L/minute and the conversion of 1 to 2 was noted as 1g/4 minutes of irradiation.
This meant a total time of 173 hours. Further processing of 2 through the double Favourskii ring contraction required significant development but eventually delivered the di-sodium salt corresponding to the di-ester of cubane.
One needs to be careful with these cubanes as they are, due to the highly strained nature of the system quite energetic materials, the do-acid and ester being more stable than the parent hydrocarbon. However the energy released upon warming above the melting point is not insignificant.
This paper represents a good demonstration of the scale-up of several very difficult chemical reactions, including excellent descriptive paragraphs of the problems and solutions. They are to be congratulated on a very nice piece of work.
See below

A scalable process for the preparation of high purity dimethyl 1,4-cubanedicarboxylate (3) is reported.
The work described herein builds on previous synthetic work from this and other laboratories, to provide a reliable process that can be used to prepare multigram quantities of 3 in a partially telescoped, 8 step process, with minimal purification of intermediates.
CSIRO Materials Science & Engineering, Ian Wark Laboratory, Bayview Avenue, Clayton Victoria 3168,Australia
Org. Process Res. Dev., 2013, 17 (12), pp 1503–1509
DOI: 10.1021/op400181g
Publication Date (Web): November 8, 2013

Scheme 5. Pilot-Scale Synthesis of Dimethyl 1,4-Cubanedicarboxylate (3)
Step 5
A dry 100 L glass reactor was charged with the crude diacid 2 (1287 g), dry methanol (36 L), and Dowex ion-exchange resin 50WX8–100 (176 g) that was prewashed with 1 L of methanol. This mixture was then stirred (150 rpm), and heated under reflux for 18 h under an atmosphere of nitrogen. The mixture was then cooled to room temperature and filtered to remove the resin. The methanol solution mixture was then evaporated to dryness using a rotary evaporator (45 °C at 45 mmHg) leaving behind the crude diester 3 (863 g) as a dark brown solid. Purification by sublimation (100–120 °C/0.01 mmHg), followed by recrystallization from acetonitrile furnished the diester 3 (560 g, 30%), as a colorless solid,
mp 164.5 °C (lit. 161–162 °C).(47)
1H NMR δ:
3.7, s, 6H TWO METHYLS
4.24, s, 6H, ring protons.
13C NMR δ:
47.03, CH3
51.55, CH
55.77, C-C=O
171.89. C=O


In fact, the single glass reactor is the most important on of all glass reactors that may include many different types such as double layer reactor, and see here: www.toption-china.com/single-glass-reactor, finner layer reactor, chemical reactor or other types of reactors.
ReplyDelete