The mass spectrum displays a molecular ion, an m-45 and a peak at m/e = 91, all of which are consistent with a molecule containing benzyl and ethoxy groups.
The 13C spectrum contains six peaks, indicating that the molecule has some elements of symmetry. The quartet at  56 and the triplet at
56 and the triplet at  71 represent a CH3 and a CH2 group which are deshielded by electronegative atoms (most likely oxygen); the peaks at
71 represent a CH3 and a CH2 group which are deshielded by electronegative atoms (most likely oxygen); the peaks at  161 - 128 are in the aromatic region; the fact that three doublets and one singlet are observed strongly suggests monosubstitution.
161 - 128 are in the aromatic region; the fact that three doublets and one singlet are observed strongly suggests monosubstitution.
 56 and the triplet at
56 and the triplet at  71 represent a CH3 and a CH2 group which are deshielded by electronegative atoms (most likely oxygen); the peaks at
71 represent a CH3 and a CH2 group which are deshielded by electronegative atoms (most likely oxygen); the peaks at  161 - 128 are in the aromatic region; the fact that three doublets and one singlet are observed strongly suggests monosubstitution.
161 - 128 are in the aromatic region; the fact that three doublets and one singlet are observed strongly suggests monosubstitution.
The proton NMR also shows evidence for two ethyl groups, a CH-CH2- group, and a monosubstituted aromatic group; the chemical shift suggests that the carbons of the CH-CH2- adjacent to one or more electronegative groups.
The IR is consistent with an aromatic compound containing a carbonyl group.
The simplest structure which is consistent with all of these data would be an aromatic compound linked via a -CH2CH group to a diethyl ester.
IUPAC Name: ethyl ethyl benzylpropanedioate (diethyl benzylmalonate)
607-81-8
C14H18O4; MW = 250.29
1H NMR
The proton NMR has a coupled quartet and a triplet, consistent with an ethyl group in which the CH2 (at  4.1) is adjacent to an electronegative atom (most likely oxygen). The presence of a coupled triplet and doublet suggests the presence of a CH-CH2- group in which both carbons are adjacent to one or more electronegative atoms. The singlet at
4.1) is adjacent to an electronegative atom (most likely oxygen). The presence of a coupled triplet and doublet suggests the presence of a CH-CH2- group in which both carbons are adjacent to one or more electronegative atoms. The singlet at  7.1 is consistent with a monosubstituted aromatic compound.
7.1 is consistent with a monosubstituted aromatic compound.
 4.1) is adjacent to an electronegative atom (most likely oxygen). The presence of a coupled triplet and doublet suggests the presence of a CH-CH2- group in which both carbons are adjacent to one or more electronegative atoms. The singlet at
4.1) is adjacent to an electronegative atom (most likely oxygen). The presence of a coupled triplet and doublet suggests the presence of a CH-CH2- group in which both carbons are adjacent to one or more electronegative atoms. The singlet at  7.1 is consistent with a monosubstituted aromatic compound.
7.1 is consistent with a monosubstituted aromatic compound.
13C NMR
The 13C spectrum contains nine peaks, indicating that the molecule has some elements of symmetry. The quartet at  14 and the triplet at
14 and the triplet at  60 represent a simple CH3 and a CH2 which is deshielded by an electronegative atom (most likely oxygen); the doublet at
60 represent a simple CH3 and a CH2 which is deshielded by an electronegative atom (most likely oxygen); the doublet at  58 and the triplet at
58 and the triplet at  36 are CH and CH2 groups which are adjacent to one or more electronegative groups. The peaks at
36 are CH and CH2 groups which are adjacent to one or more electronegative groups. The peaks at  141 - 125 are in the aromatic region; the fact that three doublets and one singlet are observed strongly suggests monosubstitution.
141 - 125 are in the aromatic region; the fact that three doublets and one singlet are observed strongly suggests monosubstitution.
 14 and the triplet at
14 and the triplet at  60 represent a simple CH3 and a CH2 which is deshielded by an electronegative atom (most likely oxygen); the doublet at
60 represent a simple CH3 and a CH2 which is deshielded by an electronegative atom (most likely oxygen); the doublet at  58 and the triplet at
58 and the triplet at  36 are CH and CH2 groups which are adjacent to one or more electronegative groups. The peaks at
36 are CH and CH2 groups which are adjacent to one or more electronegative groups. The peaks at  141 - 125 are in the aromatic region; the fact that three doublets and one singlet are observed strongly suggests monosubstitution.
141 - 125 are in the aromatic region; the fact that three doublets and one singlet are observed strongly suggests monosubstitution.
13C NMR Assignments: 
MASS
The mass spectrum consists of a molecular ion at 250, a base peak at 91 (a benzyl group), an m-45 peak at 205, indicating the presence of an ethoxy group; other significant peaks at 131 and 176 must be consistent with the proposed structure. The spectrum is consistent with a molecule containing ethoxy and benzyl groups.
Mass Spectrum Fragments: 
IR
3400-3200 cm-1: no OH peak 3100 cm-1: small peak, suggesting unsaturated CH 2900 cm-1: strong peak suggesting saturated CH 2200 cm-1: no unsymmetrical triple bonds 1730 cm-1: strong carbonyl 1610 and 1500 cm-1: weak peaks, vaguely consistent with aromatic carbon-carbon double bonds
ADDITIONAL INFO FOR READER ON SPECTROSCOPY
SEE THE FUN WHEN A CARBONYL IS INTRODUCED
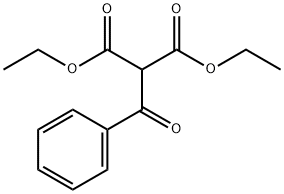
1H NMR

13 C NMR

IR

MASS

RAMAN

No comments:
Post a Comment