Protons attached to simple vinyl groups resonate from 4.5 to 7 ppm. Part of the explanation is that sp2carbons are more electronegative, since the bond contains more s character (33% compared to 25%). The carbon s orbital pulls bonds in tighter, away from the attached protons.
By itself this does not explain such a large shift. A greater effect is magnetic anisotropy. Anisotropy also explains why acetylenic protons are found from 3 to 2 ppm, since we would expect them to be higher than vinyl protons based on hybridisation alone. It also explains why the chemical shift of benzylic protons is so high.
An anisotropic field is a field that is not isotropic. An isotropic field either has a uniform density, or a spherically symmetric density distribution.
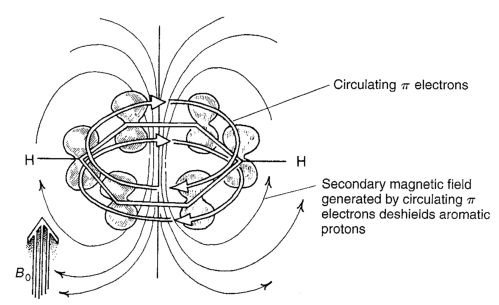
When aromatic molecules are placed in a magnetic field, mobile pi electrons in it are induced to circulate around, which itself produces a new magnetic field - an anisotropic one.
The benzylic protons are in the region deshielded by the induced anisotropic field. Protons above the ring would be shielded by the induced anisotropic field, as shown empirically in these molecules:
A similar effect is found in pi bonds, with the current moving around the internuclear axis:
For double bonds, this deshields the end protons. For triple bonds it shields them:
So an aromatic molecule in an NMR machine experiences three different magnetic fields. The external field of the machine, the diamagnetic shielding from valence electrons (an isotropic field that counteracts the external field) and any anisotropic fields induced by currents induced by the external field. The chemical shift of a proton environment depends on the net effect of these.
These opens a lot of questions such as:
Why do magnetic fields induce current in some systems but not others?
Why does the moving current itself create another magnetic field?
Why are pi electrons more mobile?
Why do pi electrons in triple bonds induce in the opposite direction to double bonds?
And so on. The answers, if any, are found in physics textbooks. Spectroscopy textbooks will just summarize what happens and what the effects are.
.pdf+-+SumatraPDF_2012-12-20_00-45-54.png)
.pdf+-+SumatraPDF_2012-12-20_00-47-58.png)
.pdf+-+SumatraPDF_2012-12-20_00-50-32.png)
.pdf+-+SumatraPDF_2012-12-20_00-50-05.png)
.pdf+-+SumatraPDF_2012-12-20_00-50-21.png)
.pdf+-+SumatraPDF_2012-12-20_01-04-21.png)
.pdf+-+SumatraPDF_2012-12-20_01-06-29.png)
No comments:
Post a Comment