
D. Srinivasa Reddy of CSIR-National Chemical Laboratory Pune devised (J. Org. Chem. 2013, 78, 8149. DOI: 10.1021/jo401033j) a cascade protocol of Diels-Alder cycloaddition of 8 to the diene 7 followed by intramolecular aldol condensation, to give the enone 9. Oxidative manipulation followed by methylenation completed the synthesis of the commercially important grapefruit flavor Nootkatone (10).

A simple and efficient synthesis of functionalized cis-hydrindanes and cis-decalins
was achieved using a sequential Diels–Alder/aldol approach in a highly
diastereoselective manner. The scope of this method was tested with a
variety of substrates and was successfully applied to the synthesis of
two natural products in racemic form. The highlights of the present work
provide ready access to 13 new cis-hydrindanes/cis-decalins,
a protecting group-free total synthesis of an insect repellent
Nootkatone, and the first synthesis of a Noreremophilane using the
shortest sequence.
A simple and efficient synthesis of functionalized cis-hydrindanes and cis-decalins was achieved using a sequential Diels–Alder/aldol approach in a highly diastereoselective manner. The scope of this method was tested with a variety of substrates and was successfully applied to the synthesis of two natural products in racemic form. The highlights of the present work provide ready access to 13 new cis-hydrindanes/cis-decalins, a protecting group-free total synthesis of an insect repellent Nootkatone, and the first synthesis of a Noreremophilane using the shortest sequence.
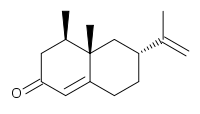
(4R*,4aS*,6R*)-4,4a-Dimethyl-6-(prop-1-en-2-yl)-4,4a,5,6,7,8-hexahydronaph thaen-2(3H)-one ((±)-Nootkatone 20)
(±)-Nootkatone 20 (19 mg, 65%).
IRυmax(film) 2923, 1668, 1606, 1459 cm–1;
1H NMR (400 MHz, CDCl3) δ 5.77 (s, 1 H), 4.74 (s, 1 H), 4.72(s, 1 H), 2.50 (ddt, J = 15.3, 5.0, 1.8 Hz, 1 H), 2.40–2.24 (m, 4 H), 2.04–1.89 (m, 3 H),1.74 (s, 3 H), 1.40–1.29 (m, 2 H), 1.11 (s, 3 H), 0.96 (d, J = 6.7 Hz, 3 H);
13C NMR (100 MHz, CDCl3) δ 199.9, 170.7, 149.3, 124.8, 109.4, 44.0, 42.2, 40.6, 40.5, 39.5, 33.2, 31.7, 21.0, 17.0, 15.0.
CAS NO. 4674-50-4, NOOTKATONE H-NMR spectral analysis predicted
CAS NO. 4674-50-4, NOOTKATONE C-NMR spectral analysis
 | |
| Names | |
|---|---|
| IUPAC name
4-α,5-Dimethyl-1,2,3,4,4α,5,6,7-octahydro-7-keto-3-isopropenylnaphthalene
| |
| Other names
(+)-nootkatone
| |
| Identifiers | |
| CAS number | 4674-50-4 |
| ChEMBL | ChEMBL446299 |
| ChemSpider | 1064812 |
| Jmol-3D images | Image |
| KEGG | C17914 |
| PubChem | 1268142 |
| Properties | |
| C15H22O | |
| Molar mass | 218.33 g·mol−1 |
| Appearance | Viscous yellow in its liquid form |
| Density | 0.968 g/mL |
| Melting point | 36 °C (97 °F; 309 K) |
| Boiling point | 170 °C (338 °F; 443 K) |
| Hazards | |
| S-phrases | S23 S24 S25 |
| Flash point | ~ 100 °C (212 °F) |
Nootkatone is a natural organic compound and is the most important and expensive aromatic of grapefruit.[1] It is a sesquiterpeneand a ketone.
Nootkatone was previouslythought to be one of the main chemical components of the smell and flavour of grapefruits. In its solid form it is usually found as crystals. As a liquid, it is viscous and yellow. Nootkatone is typically extracted from grapefruit, but can also be manufactured with genetically modified organisms, or through the chemical or biochemical oxidation of valencene. It is also found in Alaska yellow cedar trees[2] and vetiver grass.[3]
Uses
Nootkatone in spray form has been shown as an effective repellent/insecticide against deer ticks[3][4][5] and lone star ticks.[4][5] It is also an effective repellent/insecticide against mosquitos, and may repel bed bugs, head lice and other insects.[6] It is environmentally friendly insecticide, because it is a volatile essential oil that does not persist in the environment.[6] It is nontoxic to humans, is an approved food additive,[6] and "is commonly used in foods, cosmetics, and pharmaceuticals".[3]The CDC has licensed patents to two companies to produce an insecticide and an insect repellant.[6] Allylix, of San Diego, CA, is one of these licensees [7] and has developed an enzyme fermentation process that will produce nookatone more cost effectively.[8]
References
- Furusawa, Mai; Toshihiro Hashimoto; Yoshiaki Noma; Yoshinori Asakawa (November 2005). "Highly Efficient Production of Nootkatone, the Grapefruit Aroma from Valencene, by Biotransformation". Chem. Pharm. Bull. 53 (11): 1513–1514. doi:10.1248/cpb.53.1513.PMID 16272746.
- Panella, NA.; Dolan, MC.; Karchesy, JJ.; Xiong, Y.; Peralta-Cruz, J.; Khasawneh, M.; Montenieri, JA.; Maupin, GO. (May 2005). "Use of novel compounds for pest control: insecticidal and acaricidal activity of essential oil components from heartwood of Alaska yellow cedar.". J Med Entomol 42 (3): 352–8. doi:10.1603/0022-2585(2005)042[0352:UONCFP]2.0.CO;2. PMID 15962787.
- Jan Suszkiw (January 2011). "Lignin + Nootkatone = Dead Ticks". USDA.
- Dolan, MC.; Jordan, RA.; Schulze, TL.; Schulze, CJ.; Manning, MC.; Ruffolo, D.; Schmidt, JP.; Piesman, J.; Karchesy, JJ. (Dec 2009). "Ability of two natural products, nootkatone and carvacrol, to suppress Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) in a Lyme disease endemic area of New Jersey". J Econ Entomol 102 (6): 2316–24. doi:10.1603/029.102.0638. PMID 20069863.
- Jordan, Robert A.; Schulze, Terry L.; Dolan, Marc C. (January 2012). "Efficacy of Plant-Derived and Synthetic Compounds on Clothing as Repellents Against Ixodes scapularis andAmblyomma americanum (Acari: Ixodidae)". Journal of Medical Entomology 49 (1): 101–106. doi:10.1603/ME10241. PMID 22308777.
- Richard Knox (April 18, 2011). "Repelling Bugs With The Essence Of Grapefruit". NPR.
- Bigelow, Bruce (2011-04-28). "Nootkatone, So A-peeling in Grapefruit, is Repellent to Mosquitoes and Ticks". xconomy.com. Retrieved 10 August 2012.
- "Cost effective fermentation replaces costly exration". Allylix. Retrieved 10 August 2012.
External links

Dr. D. Srinivasa Reddy
https://www.linkedin.com/pub/d-srinivasa-reddy-dsreddy/1/75a/139Research areas
- Total Synthesis
- Medicinal Chemistry

DEC2014 NCL PUNE INDIA
DR ANTHONY WITH DR REDDY
Contact
- Dr. D. Srinivasa Reddy
Senior Scientist
Office: R.No-282, Main building
Organic Chemistry Division
National Chemical Laboratory
Dr. Homi Bhabha Road
Pune 411008, India
Phone +91 20 2590 2445
Fax +91 20 2590 2624
E-mail ds.reddy@ncl.res.in
Reference:
Furusawa, Mai; Hashimoto, Toshihiro; Noma, Yoshiaki; Asakawa, Yoshinori Chemical and Pharmaceutical Bulletin, 2005 , vol. 53, # 11 p. 1513 - 1514
Furusawa, Mai; Hashimoto, Toshihiro; Noma, Yoshiaki; Asakawa, Yoshinori
Chemical and Pharmaceutical Bulletin, 2006 , vol. 54, # 6 p. 861 - 868
Sauer, Anne M.; Crowe, William E.; Henderson, Gregg; Laine, Roger A.
Organic Letters, 2009 , vol. 11, # 16 p. 3530 - 3533
No comments:
Post a Comment