Xalkori, crizotinib, (PF-02341066)
3-[(1R)-1-(2,6-dichloro-3-fluorophenyl)ethoxy]-5-(1-piperidin-4-ylpyrazol-4-yl)pyridin-2-amine Crizotinib; 877399-52-5; Xalkori; PF-2341066; PF-02341066; (R)-crizotinib; 877399-52-5
| Molecular Formula: | C21H22Cl2FN5O |
|---|---|
| Molecular Weight: | 450.336683 g/mol |
The ALK-positive variation, which comprises between 3% and 5% of all NSCLC tumors, must be proved by a biomarker test. Pfizer said China's approval came just eleven months after it submitted a new drug application to the SFDA for Xalkori Crizotinib (trade name Xalkori,[1] Pfizer), is an anti-cancer drug acting as an ALK (anaplastic lymphoma kinase) and ROS1 (c-ros oncogene 1) inhibitor, approved for treatment of some non-small cell lung carcinoma (NSCLC) in the US and some other countries, and undergoing clinical trials testing its safety and efficacy in anaplastic large cell lymphoma, neuroblastoma, and other advanced solid tumors in both adults and children.[2]
- FDA approves Xalkori with companion diagnostic for a type of late-stage lung cancer. U.S. Food and Drug Administration.http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm269856.htm
- ClinicalTrials.gov NCT00932451 An Investigational Drug, PF-02341066, Is Being Studied In Patients With Advanced Non-Small Cell Lung Cancer With A Specific Gene Profile Involving The Anaplastic Lymphoma Kinase (ALK) Gene
.
4 .
........................
http://www.specchemonline.com/articles/view/biocatalyst-breakthroughs#.VTcW9yxabEs
.........................
http://www.google.com/patents/WO2014020467A2?cl=en
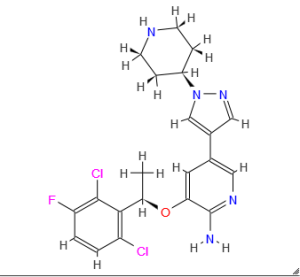
(R)-3-[l-(2,6-Dichloro-3-fluoro-phenyl)-ethoxy]-5-(l-piperidin-4-yl-lH-py- razol-4-yl)-pyridin-2-ylamine, also known as Crizotinib, is represented by the Formula (I):
Formula (I) Crizotinib is a potent small-molecule inhibitor of c-Met/HGFR (hepatocyte growth factor receptor) kinase and ALK (anaplastic lymphoma kinase) activity. Enantiomerically pure compound of formula I was first disclosed in US Patent No. 7,858,643. Additionally, the racemate of compound of formula I was disclosed in U.S. patent application 2006/0128724, both of these references discloses similar methods for the synthesis of Compound of Formula I.
Conventionally, the compounds of formula I are prepared by reacting Bis(pinacolato)diboron with protected 5-bromo-3-[l-(2,6-dichloro-3-fluoro-phenyl)-ethoxy]-pyridin-2-ylamine in the presence of Pd catalyst. The obtained product after deprotection is reacted with N- protected 4-(4-bromo-pyrazol-l-yl)-piperidine in the presence of Pd Catalyst. The obtained product is filtered through celite pad and purified by Column Chromatography. The final product of formula I was obtained by deprotection of the purified compound by using HCl/dioxane.
US Patent No. 7,858,643 provides enantiomerically pure aminoheteroaryl compounds, particularly aminopyridines and aminopyrazines, having protein tyrosine kinase activity. More particularly, US 7,858,643 describes process for the preparation of 3-[(lR)-l-(2,6- dichloro-3-fluorophenyl)ethoxy]-5-(l-piperidin-4-ylpyrazol-4-yl)pyridin-2-amine. The Scheme is summarized below in Scheme- 1 :
Scheme-1 wherein, "Boc" means tert-butoxycarbonyl; and a) (Boc)2, DMF, Dimethylaminopyridine b) Pd(dppf)Cl2, KOAc, Dichloromethane; c) HC1, Dioxane, Dichloromethane; d) Pd(PPh3)2Cl2, Na2C03, DME/H20; e) 4M HCl/Dioxane, Dichloromethane A similar process has been disclosed in the U.S. patent application 2006/0128724 for the preparation of Crizotinib. J. Jean Cui et. al. in J. Med. Chem. 2011, 54, 6342-6363, also provides a similar process for the preparation of Crizotinib and its derivatives. However, above mentioned synthetic process requires stringent operational conditions such as filtration at several steps through celite pad. Also column chromatography is required at various steps which is not only tedious but also results in significant yield loss. Another disadvantage of above process involves extensive use of palladium catalysts, hence metal scavengers are required to remove palladium content from the desired product at various steps which makes this process inefficient for commercial scale.
Yet another disadvantage of above process is the cost of Bis(pinacolato)diboron. This reagent is used in excess in the reaction mixture resulting in considerable cost, especially during large-scale syntheses. US Patent No. 7,825,137 also discloses a process for the preparation of Crizotinib where Boc protected 4-(4-iodo-pyrazol-l-yl)-piperidine is first reacted with Bis(pinacolato)diboron in the presence of Pd catalyst.
The reaction mixture is filtered through a bed of celite and the obtained filtrate is concentrated and purified by silica gel chromatography to give to form tert-butyl-4-[4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)-lH-pyrazol-l-yl]piperidine-l- carboxylate. To this compound, 5-bromo-3-[l-(2,6-dichloro-3-fluoro-phenyl)-ethoxy]- pyridin-2-ylamine is added in the presence of a Pd catalyst. The reaction mixture is stirred for 16h at 87°C.
The reaction mixture is filtered through celite pad and the concentrated filtrate is purified on silica gel column to obtain (4-{6-amino-5-[(R)-l-(2,6-dichloro-3-fluoro- phenyl)-ethoxy]-pyri- din-3-yl}-pyrazol-l-yl)-piperidine-l-carboxylic acid tert-butyl ester of 95% purity. To the solution of resulting compound in dichloromethane 4N HCl/Dioxane is added and thereby getting the reaction suspension is filtered in Buchner funnel lined with filter paper.
The obtained solid is dissolved in HPLC water and pH is adjusted to 10 with the addition of Na2C03 Compound is extracted using dichloroform and is purified on a silica gel column by eluting with CH2Cl2 MeOH/NEt3 system to obtain Crizotinib. The scheme is summarized below in scheme 2:
Formula (i) Formula (ii)
Formula (iii) Formula (ii) ula (iv)
Formula (v) Formula (I) Scheme-2
Preparation of Crizotinib:
To a stirred solution of Tert-butyl 4-(4-{ 6-amino-5-[(li?)-l-(2,6-dichloro-3- fluorophenyl)ethoxy]pyridin-3 -yl } - lH-pyrazol- 1 -yl)piperidine- 1 -carboxylate (material obtained in Example 3) (l.Og, 0.00181 moles) in dichloromethane (-13 ml) at 0°C was added 4.0 M dioxane HQ (6.7 ml, 0.0272 moles). Reaction mixture was stirred at room temperature for 4h. After the completion of reaction monitored by TLC, solid was filtered and washed with dichloromethane (10 ml).
The obtained solid was dissolved in water (20 ml); aqueous layer was extracted with dichloromethane (10x2). The pH of aqueous layer was adjusted to 9-10 with Na2C03 and compound was extracted with dichloromethane (10 x 3), combined organic layers were washed with water (20 ml), evaporated under vacuum to get solid product. The solid was stirred with ether (10 ml), filtered off, washed well with ether, dried under vacuum to get Crizotinib. Yield: 0.45g (55 %) HPLC Purity: 99.35 %
1HNMR (400 MHz, CDC13) δ: 7.76 (d, J = 1.6 Hz, 1H), 7.56 (s, 1H), 7.49 (s, 1H), 7.30 (dd, J = 9.2 Hz), 7.0 (m, 1H), 6.86 (d, J = 1.6 Hz, 1H), 6.09 ( q, J= 6.8 Hz, 1H), 4.75 (brs, 1H), 4.19 (m, 1H), 3.25 (m, 2H), 2.76 (m, 2H), 2.16 (m, 2H), 1.92 (m, 2H), 1.85 (d, J= 6.8 Hz, 3H), 1.67 (brs, 1H)
..............................
http://www.sciencedirect.com/science/article/pii/S0040403914000872
Abstract
A novel approach for the synthesis of Crizotinib (1) is described. In addition, new efficient procedures have been developed for the preparation of (S)-1-(2,6-dichloro-3-fluorophenyl)ethanol (2) and tert-butyl 4-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)piperidine-1-carboxylate (4), the key intermediates required for the synthesis of Crizotinib.
Graphical abstract
- .....................
- http://www.sciencedirect.com/science/article/pii/S0040403911021745
-
Abstract
4-(4-Iodo-1H-pyrazol-1-yl)piperidine is a key intermediate in the synthesis of Crizotinib. We report a robust three-step synthesis that has successfully delivered multi-kilogram quantities of the key intermediate. The process includes nucleophilic aromatic substitution of 4-chloropyridine with pyrazole, followed by hydrogenation of the pyridine moiety and subsequent iodination of the pyrazole which all required optimization to ensure successful scale-up.
Org. Process Res. Dev., 2011, 15 (5), pp 1018–1026
DOI: 10.1021/op200131n
A robust six-step process for the synthesis of crizotinib, a novel c-Met/ALK inhibitor currently in phase III clinical trials, has been developed and used to deliver over 100 kg of API. The process includes a Mitsunobu reaction, a chemoselective reduction of an arylnitro group, and a Suzuki coupling, all of which required optimization to ensure successful scale-up. Conducting the Mitsunobu reaction in toluene and then crystallizing the product from ethanol efficiently purged the reaction byproduct.
A chemoselective arylnitro reduction and subsequent bromination reaction afforded the key intermediate 6. A highly selective Suzuki reaction between 6 and pinacol boronate 8, followed by Boc deprotection, completed the synthesis of crizotinib 1.
A chemoselective arylnitro reduction and subsequent bromination reaction afforded the key intermediate 6. A highly selective Suzuki reaction between 6 and pinacol boronate 8, followed by Boc deprotection, completed the synthesis of crizotinib 1.
Mp 192 °C;
1H NMR (400 MHz, CDCl3) δ: 7.78 (d, J = 1.8 Hz, 1H), 7.58 (s, 1H), 7.52 (s, 1H), 7.31 (dd, J = 9.0, 4.9 Hz, 1H), 7.06 (m, 1H), 6.89 (d, J = 1.7 Hz, 1H), 6.09 (q, 1H), 4.79 (br s, 2H), 4.21 (m, 1H), 3.26 (m, 2H), 2.78 (m, 2H), 2.17 (m, 2H), 1.90 (m, 2H), 1.87 (d, J = 6.7 Hz, 3H), 1.63 (br s, 1H).
13C NMR (100.6 MHz, CDCl3) δ: 157.5 (d, J = 250.7 Hz), 148.9, 139.8, 137.0, 135.7, 135.6, 129.9, 129.0 (d, J = 3.7 Hz), 122.4, 122.1 (d, J = 19.0 Hz), 119.9, 119.3, 116.7 (d, J = 23.3 Hz), 115.0, 72.4, 59.9, 45.7, 34.0, 18.9.
LC-MS: found m/z 450.0, 451.0, 452.0, 453.0, 454.0, 455.0. Anal. Calcd for C21H22Cl2FN5O: C, 56.01; H, 4.92; N, 15.55. Found: C, 56.08; H, 4.94; N, 15.80.
Cui, J. J.; Botrous, I.; Shen, H.; Tran-Dube, M. B.; Nambu, M. D.; Kung, P.-P.; Funk, L. A.; Jia, L.; Meng, J. J.; Pairish, M. A.; McTigue, M.; Grodsky, N.; Ryan, K.; Alton, G.; Yamazaki, S.; Zou, H.; Christensen, J. G.; Mroczkowski, B.Abstracts of Papers; 235th ACS National Meeting, New Orleans, LA, United States, April 6–10, 2008.
Cui, J. J.; Funk, L. A.; Jia, L.; Kung, P.-P.; Meng, J. J.; Nambu, M. D.; Pairish, M. A.; Shen, H.; Tran-Dube, M. B. U.S. Pat. Appl. U. S. 2006/0046991 A1, 2006.
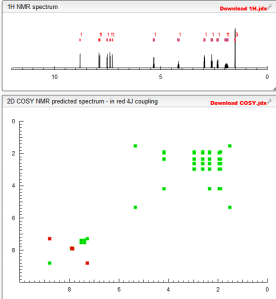
Cosy predict above
1H NMR PREDICT
13C NMR PREDICT
-
.................
WO2010048131A1 * Oct 20, 2009 Apr 29, 2010 Vertex Pharmaceuticals Incorporated C-met protein kinase inhibitors WO2011042389A2 * Oct 4, 2010 Apr 14, 2011 Bayer Cropscience Ag Phenylpyri(mi)dinylazoles US7825137 Nov 23, 2006 Nov 2, 2010 Pfizer Inc. Method of treating abnormal cell growth US7858643 Aug 26, 2005 Dec 28, 2010 Agouron Pharmaceuticals, Inc. Crizotinib, a c-Met protein kinase inhibitor anticancer agent; 3-[(R)-1-(2,6-dichloro-3-fluoro-phenyl)-ethoxy]-5-(1-piperidin-4-yl-1H-pyrazol-4-yl)-pyridin-2-ylamine is crizotinib US20060128724 Aug 26, 2005 Jun 15, 2006 Agouron Pharmaceuticals, Inc. Pyrazole-substituted aminoheteroaryl compounds as protein kinase inhibitors 1 J. JEAN CUI J. MED. CHEM. vol. 54, 2011, pages 6342 - 6363 2 ORG. PROCESS RES. DEV. vol. 15, 2011, pages 1018 - 1026 3 * PIETER D. DE KONING ET AL: "Fit-for-Purpose Development of the Enabling Route to Crizotinib (PF-02341066)", ORGANIC PROCESS RESEARCH & DEVELOPMENT, vol. 15, no. 5, 16 September 2011 (2011-09-16), pages 1018-1026, XP055078841, ISSN: 1083-6160, DOI: 10.1021/op200131n
GLIMPSES OF INDIA
http://en.wikipedia.org/wiki/Pondicherry
Pondicherry India
Panoramic view of Pondicherry town
Pondicherry waterfront circa 1900
.
Visitors looking at Mamallapuram Temples, India
Colonial quarter street
A remarkable degree of French influence in Pondicherry exists to this date. Pondicherry was designed based on the French grid pattern and features neat ...
Panoramic view of Pondicherry beach - P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.

COCK WILL TEACH YOU NMR
 COCK SAYS MOM CAN TEACH YOU NMR
COCK SAYS MOM CAN TEACH YOU NMR
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO .....FOR BLOG HOME CLICK HERE
amcrasto@gmail.com
- KALININGRAD RUSSIA
Brandenburg Gate. Kaliningrad ...

No comments:
Post a Comment