NMR Spectroscopy 1: The basics
Hello everybody!
This morning, I struggled with NMR spectroscopy. I thought I understand the theory. However, when I was asked to draw a 1H NMR Spectrum of a molecule, I couldn't do it. I wonder what is the problem. So, I thought I put it write here.
Here how it's going to go. Firstly, I am going to write in my own word the theory behind NMR Spectroscopy. Secondly, I am going to confront my problem that is drawing NMR Spectrum of a molecule. Ultimately, I want to make sure that I understand all relevant information and I am able to make use of it for whatever objectives I want to achieve depending on the question.
Before I begin, I would like to share with you the question that I have been asked.
Q: Draw the 1H NMR Spectrum you would expect for the drug molecule. Annotate clearly on the spectrum the chemical shift, integration information and splitting patterns you would expect. (5 marks)
OK. Let's begin.
Properties of nuclei.
One of the properties of nuclei is that it has nuclear spin. However, not all nuclei have it. Nuclear spin is quantized and has the symbol I. The exact number of different energy levels that a nucleus has depends on the value of I of that particular isotope. (For my purpose, we do not want to go deeper into this.) Just concentrate on 2 different nuclei that is 13C and 1H. Both have only 2 different energy levels.
NMR uses a strong magnetic field.
In an NMR instrument, there is a large supercooled magnet that provides external magnetic field. When a sample is put into the instrument, all nuclei which possess nuclear spin will be in their lowest energy state. For the case of 13C and 1H, they both have two different energy levels; one is aligned with the external magnetic field (therefore, lower energy level) and the other one is against the external magnetic field (therefore, higher energy level).
The difference between the two energy levels can be measured.
Nuclei that possess nuclear spin and be in their lowest energy state in an external field is said to interact with the magnetic field. The difference between the two energy levels can be measured (how it is done will be explained later). The difference is given as :
Radio wave is in the frequency of 10^4 Hz to 10^8 Hz. The specificity of the spectrum is determined by the frequency of the radio waves i.e. each nuclei require certain frequency of radio waves to flip it from lower energy spin state to higher energy spin state.
The sample is irradiated with short pulse of radio waves of specific frequency. After irradiation, nuclei that is promoted to higher energy spin state will return to lower energy spin state. Energy is released as the nuclei fall back down and this energy can be measured.
Measurement obtained from NMR spectroscopy has to be Fourier transformed to obtained meaningful spectrum.
This is the confusing bit but since I am not an analytical chemist nor physical chemist, we can just accept the fact that the measurement of energy released has to be mathematically treated to produce typical NMR spectrum that we pharmacist can analysed.
The following diagram is an example of a typical NMR spectrum.
At this moment, we are not going to interpret. I am just showing you the typical NMR spectrum. In reality, NMR spectrum is way more complicated than this and many research lab has been developing algorithm to improve the resolution (that is the ability to see each peak as distinct). We do not want to get deeper into this as this is for advanced study. I don't need it at the moment.
What kind of information does NMR Spectrum gives?
Fundamentally, NMR spectrum enables you to detect atomic nuclei and identify what environment it is in.
Several important information that you can gain from 1H NMR spectrum. (refer to the 1H NMR spectrum of ethanol for clarity)
Chemical shift scale
The units for abscissa (or x-axis) is parts per million. Why is this the case? This is because the frequency of each nuclei environment depends on the strength of the external magnetic field. If the sample is run on a different NMR instrument or different strength of the magnetic field, the frequency at which that nuclei resonates will be different.
Since the instrument usually has frequency of up to 900MHz and atomic nuclei resonates at frequency in Hz, it makes sense to use part per million. (Hz in MHz)
The ordinate represents relative amount of nuclei.
What gives each nuclei environment a distinct chemical shift?
Recall that we measure the energy released by nuclei as it falls down from higher energy level spin state to lower energy level spin state.
Each nuclei in a molecule resonate at different frequency. Why is this the case?
Logical deduction that we can make is is that the ΔE is difference for each nuclei. Recall that ΔE = hf.
Though the external magnetic field is constant for all nuclei, the magnetic field experienced by each nuclei is not exactly the same. .
Each nucleus is surrounded by electrons. In a magnetic field, electrons can generate tiny electrical current. Consequently, this electrical current will generate magnetic field.
When nucleus is protected by electrons around it, it is called shielding as the electrons shield the nucleus from external magnetic field. Consequently, the resonating frequency will shift upfield.
The vice versa is true. (More on this later.)
Take ethanol for example. I suggest you draw the structure in a piece of paper and follow my explanation. Oxygen atom is an electronegative atom which means in ethanol molecule electrons density will be higher towards the oxygen atom. As a result, proton attached to carbon next to oxygen atom will have less electron density hence deshielded. The nuclei will experience more external magnetic field i.e. greater energy difference. From ΔE = hf, we can infer that resonating frequency will also increase. Hence, the signal for CH2 is further downfield with regards to CH2 signal position without the oxygen atom.
OK. Are you ready to continue?
Describing chemical shift.
If you find it difficult to follow the previous concept, I think one of the reason is that I haven't introduced the terminology to describe chemical shift.
First, I want to define chemical shift. Chemical shift is the resonating frequency with respect to standard as a fraction of frequency of instrument.
When we are saying about the field i.e. magnetic field, we use downfield and upfield.
When we are saying about chemical shift, we use large or small.
When we are saying about frequency, we use high or low.
When we are saying about shielding, we use deshielded or shielded.
I think the best that I can do now is to give you example so that you understand.
So far, I would like to wrap up a few things before we continue.
Why each nuclei resonates at different frequency?
Each nuclei experience different magnetic field due to distribution of electrons around the nuclei leading to either shielding or deshielding.
What happens to resonating frequency when the nuclei is deshielded and vice versa?
Think about it this way. When a nucleus is deshielded, it experiences greater external magnetic field. Consequently, ΔE will increase. According to equation ΔE = hf, as ΔE increases, resonating frequency will also increase. Hence, the position of the signal will be further downfield.
OK. I think we are now ready to go to deeper.
Regions of the 1H NMR spectrum.
Many observations have been made and it is important to memorise the common proton environment.
Proton in:
CH3 = 1 ppm
CH2 = 2 ppm
CH = 3 ppm
Benzene = 7.2 ppm
Also, a diagram to help you remember the rough position of signal.
Our discussion on NMR spectroscopy has not finished. I am going to continue more on it tomorrow.
This morning, I struggled with NMR spectroscopy. I thought I understand the theory. However, when I was asked to draw a 1H NMR Spectrum of a molecule, I couldn't do it. I wonder what is the problem. So, I thought I put it write here.
Here how it's going to go. Firstly, I am going to write in my own word the theory behind NMR Spectroscopy. Secondly, I am going to confront my problem that is drawing NMR Spectrum of a molecule. Ultimately, I want to make sure that I understand all relevant information and I am able to make use of it for whatever objectives I want to achieve depending on the question.
Before I begin, I would like to share with you the question that I have been asked.
 |
| Structure of phenobarbitone. Source: Wikipedia |
OK. Let's begin.
Properties of nuclei.
One of the properties of nuclei is that it has nuclear spin. However, not all nuclei have it. Nuclear spin is quantized and has the symbol I. The exact number of different energy levels that a nucleus has depends on the value of I of that particular isotope. (For my purpose, we do not want to go deeper into this.) Just concentrate on 2 different nuclei that is 13C and 1H. Both have only 2 different energy levels.
NMR uses a strong magnetic field.
In an NMR instrument, there is a large supercooled magnet that provides external magnetic field. When a sample is put into the instrument, all nuclei which possess nuclear spin will be in their lowest energy state. For the case of 13C and 1H, they both have two different energy levels; one is aligned with the external magnetic field (therefore, lower energy level) and the other one is against the external magnetic field (therefore, higher energy level).
The difference between the two energy levels can be measured.
Nuclei that possess nuclear spin and be in their lowest energy state in an external field is said to interact with the magnetic field. The difference between the two energy levels can be measured (how it is done will be explained later). The difference is given as :
ΔE depends on:ΔE = hf where ΔE is the difference in energy, h is the planck constant (h = 6.62606957 × 10-34 m2 kg / s ), and f is the frequency of the radio waves.
- How strong the magnetic field is
- Magnetic properties of the nucleus itself
Radio wave is in the frequency of 10^4 Hz to 10^8 Hz. The specificity of the spectrum is determined by the frequency of the radio waves i.e. each nuclei require certain frequency of radio waves to flip it from lower energy spin state to higher energy spin state.
The sample is irradiated with short pulse of radio waves of specific frequency. After irradiation, nuclei that is promoted to higher energy spin state will return to lower energy spin state. Energy is released as the nuclei fall back down and this energy can be measured.
Measurement obtained from NMR spectroscopy has to be Fourier transformed to obtained meaningful spectrum.
This is the confusing bit but since I am not an analytical chemist nor physical chemist, we can just accept the fact that the measurement of energy released has to be mathematically treated to produce typical NMR spectrum that we pharmacist can analysed.
The following diagram is an example of a typical NMR spectrum.
| 1H NMR spectrum of ethanol. Source: Wikipedia |
What kind of information does NMR Spectrum gives?
Fundamentally, NMR spectrum enables you to detect atomic nuclei and identify what environment it is in.
Several important information that you can gain from 1H NMR spectrum. (refer to the 1H NMR spectrum of ethanol for clarity)
- The number of peaks indicate the number of proton environment.
- The area under the peak or integration information tells you how many protons are there. Note: This information is in the form of ratio. In order to correctly identify how many protons are present, other techniques like Mass Spectroscopy should be used to precisely determine number of atoms in the molecule.
- The splitting pattern indicates the number of neighbouring nuclei that the nuclei interact with. Note that if the nuclei interact, they will have the same coupling constant. (usually written as J but it is not shown in this spectrum).
- Chemical shift tells you about the nature of the environment. This is the most difficult part of interpreting an NMR spectrum. However, we are going to go through this at some point in this post. Just wait for it.
Chemical shift scale
The units for abscissa (or x-axis) is parts per million. Why is this the case? This is because the frequency of each nuclei environment depends on the strength of the external magnetic field. If the sample is run on a different NMR instrument or different strength of the magnetic field, the frequency at which that nuclei resonates will be different.
Before I continue, I would like to explain the meaning of the term resonates. In NMR spectroscopy, when nuclei is said to resonate it means that nuclei is absorbing energy from the radio waves so that it can be promoted to higher energy level spin state. As it falls down to lower energy level spin state, it releases energy which through several steps gives the NMR spectrum.Hence, it will be difficult to say exactly where our signal is. Therefore, we defined our signal position by how far it is from a reference sample (tetramethylsilane is often used as reference), as a fraction of the operating frequency of the instrument.
Since the instrument usually has frequency of up to 900MHz and atomic nuclei resonates at frequency in Hz, it makes sense to use part per million. (Hz in MHz)
The ordinate represents relative amount of nuclei.
What gives each nuclei environment a distinct chemical shift?
Recall that we measure the energy released by nuclei as it falls down from higher energy level spin state to lower energy level spin state.
Each nuclei in a molecule resonate at different frequency. Why is this the case?
Logical deduction that we can make is is that the ΔE is difference for each nuclei. Recall that ΔE = hf.
Though the external magnetic field is constant for all nuclei, the magnetic field experienced by each nuclei is not exactly the same. .
Each nucleus is surrounded by electrons. In a magnetic field, electrons can generate tiny electrical current. Consequently, this electrical current will generate magnetic field.
Basic physics guys. Just to remind myself again. Suppose we have a wire and we coil it so that it forms a solenoid. When we pass current through it, magnetic field is generated.This magnetic field which we will call local magnetic field will oppose the external magnetic field. Electron distribution around the nucleus vary for each environment. Two possible situations: shielding and deshielding.
When nucleus is protected by electrons around it, it is called shielding as the electrons shield the nucleus from external magnetic field. Consequently, the resonating frequency will shift upfield.
The vice versa is true. (More on this later.)
Take ethanol for example. I suggest you draw the structure in a piece of paper and follow my explanation. Oxygen atom is an electronegative atom which means in ethanol molecule electrons density will be higher towards the oxygen atom. As a result, proton attached to carbon next to oxygen atom will have less electron density hence deshielded. The nuclei will experience more external magnetic field i.e. greater energy difference. From ΔE = hf, we can infer that resonating frequency will also increase. Hence, the signal for CH2 is further downfield with regards to CH2 signal position without the oxygen atom.
I know things has become more difficult. Don't give up. You just need to get your head around it. I suggest you ensure that you understand everything up till this point. Read back from the beginning if necessary.
OK. Are you ready to continue?
Describing chemical shift.
If you find it difficult to follow the previous concept, I think one of the reason is that I haven't introduced the terminology to describe chemical shift.
First, I want to define chemical shift. Chemical shift is the resonating frequency with respect to standard as a fraction of frequency of instrument.
It's hard to explain the terminology without using a diagram. However, if you look into textbook of organic chemistry, you will find one.
When we are saying about the field i.e. magnetic field, we use downfield and upfield.
When we are saying about chemical shift, we use large or small.
When we are saying about frequency, we use high or low.
When we are saying about shielding, we use deshielded or shielded.
I think the best that I can do now is to give you example so that you understand.
So far, I would like to wrap up a few things before we continue.
Why each nuclei resonates at different frequency?
Each nuclei experience different magnetic field due to distribution of electrons around the nuclei leading to either shielding or deshielding.
What happens to resonating frequency when the nuclei is deshielded and vice versa?
Think about it this way. When a nucleus is deshielded, it experiences greater external magnetic field. Consequently, ΔE will increase. According to equation ΔE = hf, as ΔE increases, resonating frequency will also increase. Hence, the position of the signal will be further downfield.
OK. I think we are now ready to go to deeper.
Regions of the 1H NMR spectrum.
Many observations have been made and it is important to memorise the common proton environment.
Proton in:
CH3 = 1 ppm
CH2 = 2 ppm
CH = 3 ppm
Benzene = 7.2 ppm
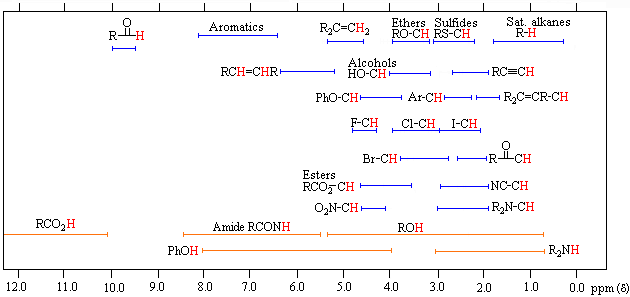
Also, a diagram to help you remember the rough position of signal.
Our discussion on NMR spectroscopy has not finished. I am going to continue more on it tomorrow.
No comments:
Post a Comment