 .
.The Catellani Reaction, a superb C-H Activation reaction. This recent review http://bit.ly/1LMNaKM outlines the challenges and future opportunities. Enjoy! #organichemistry #catellanireaction #CHactivation
LINK
http://urlaa.com/Catellani-Reaction-Perspective.pdf
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3715096/
lignan (+)-linoxepin 1 using domino C–H functionalization with an overall yield of 30 %. This synthesis is the first reported application of the palladium-catalyzed Catellani reaction in the synthesis of a complex natural product. We note that the optical rotation of the synthetic material is higher (
Synthesis of (+)-linoxepin (1). a) PdCl2 (20 mol %), PPh3 (44 mol %), CsOAc (10.0 equiv), DMF, 75 °C, 4 h, 76 %.
ACADEMIC........................
The Catellani reaction was devised by Marta Catellani. It is Norbornene-mediated Ortho C-H functionalization consisting series of reaction.[1] Norbornene acts as a catalyst in this reaction.[2]

Reaction mechanism
Catellani reaction consist of a series of reaction and Norbornene acts as the catalyst in this reaction along with Palladium.[3] Rhodium can also be used as a catalyst along with Norbornene instead of Palladium.[4]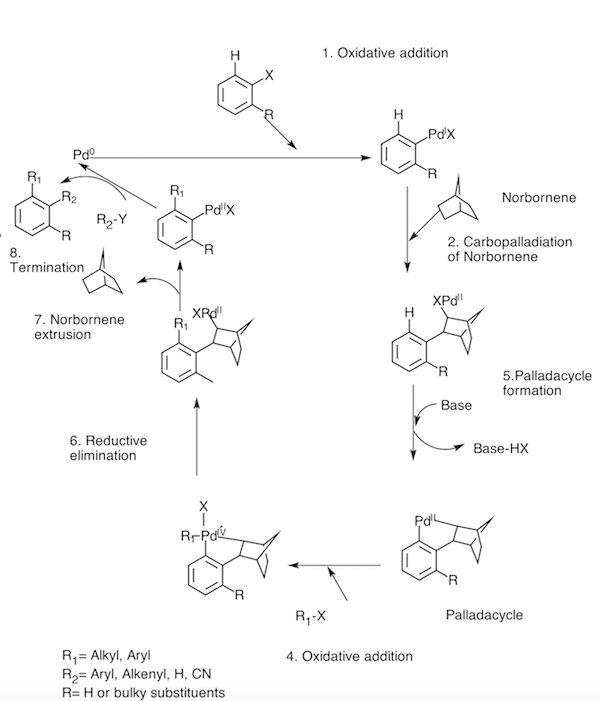
Different Steps of catellani reaction: 1. Oxidative addition 2. Carbopalledation with Norbornene 3. Palladocycle formation 4. Oxidative addition 5. Reductive elimination 6. Norbornene extrusion 7. Termination
Uses
Catellani reaction is used for polyfunctionalization of aromatic molecule. It has been used as a key step for synthesis of novel lignan (+)- linoxepin.[5] It can also be used for synthesis of Rhazinal.[6]References
- Sui; et al. (June 2013). "Pd-Catalyzed Chemoselective Catellani Ortho-Arylation of Iodopyrroles: Rapid Total Synthesis of Rhazinal". J. Am. Chem. Soc 135 (25): 9318–9321. doi:10.1021/ja404494u. Retrieved 26 December 2014.
External links
MORE........
http://www.rsc.org/suppdata/cc/c3/c3cc46381h/c3cc46381h_2.pdf
[PDF]Catellani reaction experimental revised - Royal Society of ...
www.rsc.org/suppdata/cc/c3/c3cc46381h/c3cc46381h_2.pdf
//////////
No comments:
Post a Comment