Atorvastatin calcium
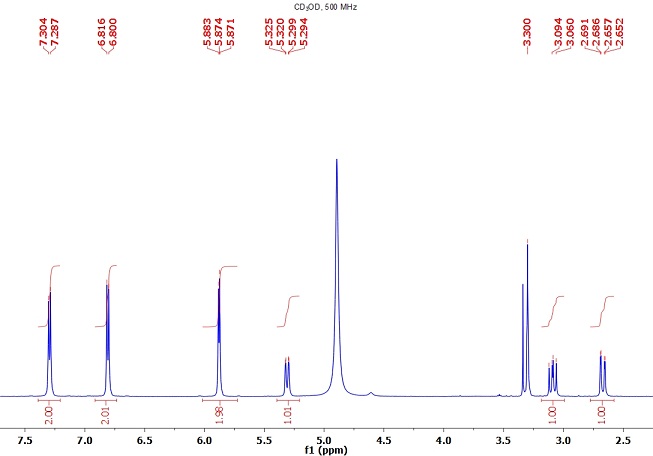
1H NMR DMSOD6
13C NMR DMSOD6
.....................................
http://www.google.com/patents/EP0848705B1?cl=en
- [0028]All solid-state 13C NMR measurements were made with a Bruker AX-250, 250 MHz NMR spectrometer. High resolution spectra were obtained using high-power proton decoupling and cross-polarization (CP) with magic-angle spinning (MAS) at approximately 5 kHz. The magic-angle was adjusted using the Br signal of KBr by detecting the side bands as described by Frye and Maciel (Frye J.S. and Maciel G.E., J. Mag. Res., 1982;48:125). Approximately 300 to 450 mg of sample packed into a canister-design rotor was used for each experiment. Chemical shifts were referenced to external tetrakis (trimethylsilyl)silane (methyl signal at 3.50 ppm) (Muntean J.V. and Stock L.M., J. Mag. Res., 1988;76:54).
- [0029]Table 2 shows the solid-state NMR spectrum for crystalline Form I atorvastatin hydrate.
Carbon Atom Assignment and Chemical Shift for Form I Atorvastatin hydrate Assignment (7 kHz) Chemical Shift C12 or C25 182.8 C12 or C25 178.4 C16 166.7 (broad) and 159.3 Aromatic Carbons C2-C5, C13-C18, C19-C24, C27-C32 137.0 134.9 131.1 129.5 127.6 123.5 120.9 118.2 113.8 C8,C10 73.1 70.5 68.1 64.9 Methylene Carbons C6, C7, C9, C11 47.4 41.9 40.2 C33 26.4 25.2 C34 21.3
..................
http://www.google.co.in/patents/US7834195
Atorvastatin calcium (5 g) was dissolved in racemic propylene glycol followed by the addition of 7 parts of ethyl acetate. The resulting mixture was warmed to 55-60° C. and stirred for 8-10 hours to afford a white suspension. The suspension was cooled to 20-25° C. and filtered to provide 3.3 g of atorvastatin calcium propylene glycol solvate after drying under vacuum at 50-60° C. Propylene glycol content: 6% by NMR.
Isopropyl acetate and methyl, isobutyl ketone (MIBK) can also be used in the example 1 procedure.
The DSC and IR of the solvate made in this example is shown in FIGS. 1 and 2 , respectively.
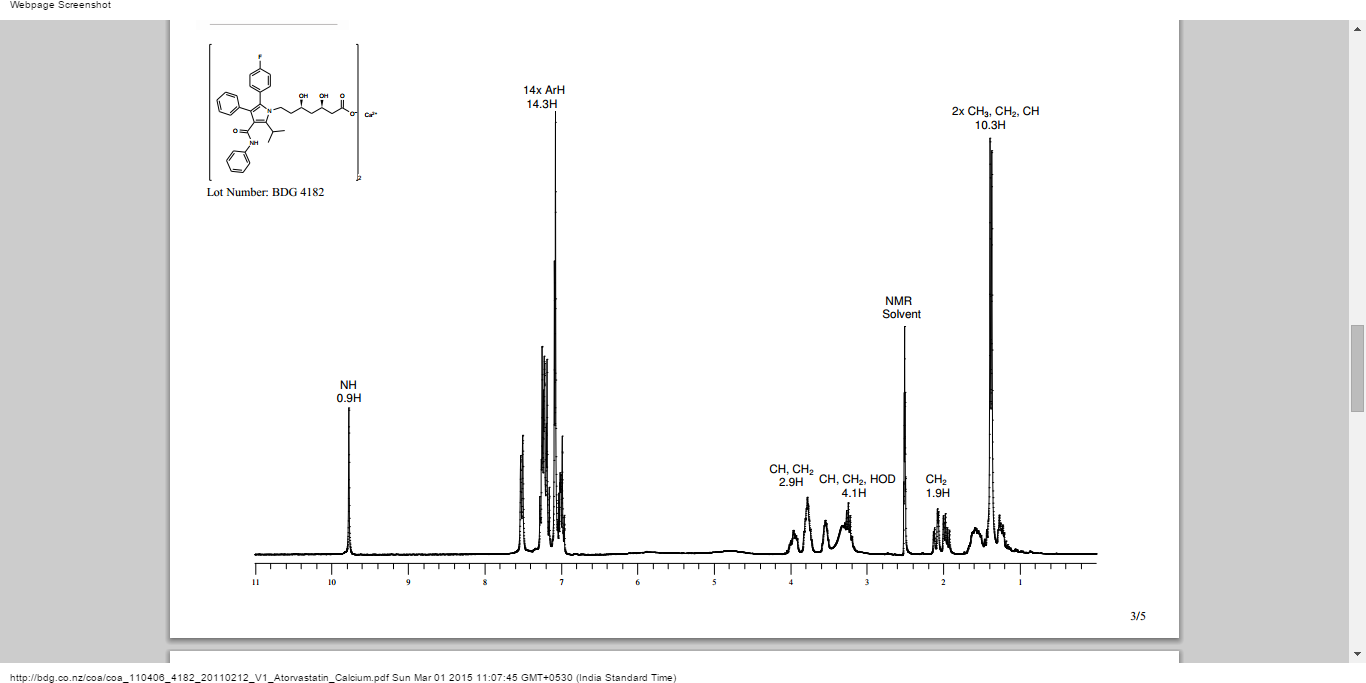
1H-NMR(DMSO-d6): δ 9.82 (s, 1H), 7.51 (ad, J=8.0 Hz, 2H), 7.33-7.11 (m, 6H), 7.08-6.95 (m, 6H), 5.93 (bs, 1H), 4.76 (bs, 1H), 4.65-4.33 (m, 1H), 4.09-3.85 (m, 1H), 3.84-3.68 (m, 2H), 3.62-3.44 (m, 1.5H), 3.30-3.09 (m, 2H), 2.08 (dd, J=15.4, 3.7 Hz), 1.97 (dd, J=15.3, 8.0 Hz), 1.71-1.50 (m, 2H), 1.50-1.31 (m, 7H), 1.30-1.11 (m, 1H), 1.00 (d, J=6.3 Hz, 1.5H).
http://www.google.st/patents/US20040054193
urther, the present invention is directed to crystalline Form V atorvastatin and hydrates thereof characterized by the following solid-state 13C nuclear magnetic resonance (ssNMR) spectrum wherein chemical shift is expressed in parts per million:
| Assignment | Chemical Shift | ||
| C12 or C25 | 185.7 | ||
| C12 or C25 | 176.8 | ||
| C16 | 166.9 | ||
| Aromatic Carbons | 138.7 | ||
| C2-C5, C13-C18, | 136.3 | ||
| C19-C24, C27-C32 | |||
| 133.0 | |||
| 128.4 | |||
| 122.0 | |||
| 117.0 | |||
| 116.3 | |||
| C8, C10 | 68.0 | ||
| Methylene Carbons | 43.1 | ||
| C6, C7, C9, C11 | |||
| C33 | 25.6 | ||
| C34 | 19.9 | ||
[0017] Additionally, the present invention is directed to crystalline Form V atorvastatin and hydrates thereof characterized by the following Raman spectrum having peaks expressed in cm−1:
| 3062 |
| 1652 |
| 1604 |
| 1528 |
| 1478 |
| 1440 |
| 1413 |
| 1397 |
| 1368 |
| 1158 |
| 1034 |
| 1001 |
| 825 |
| 245 |
| 224 |
| 130 |
DACCA, BANGLADESH
Farmer Harvesting Jute, Tangail,Dhaka, Bangladesh Photographic Print
////////////
.png)



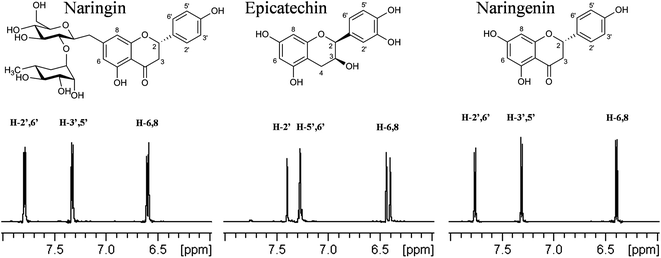
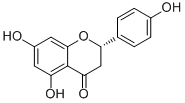 Aldrich Library of 13C and 1H FT NMR Spectra, 1992, 2, 915C
Aldrich Library of 13C and 1H FT NMR Spectra, 1992, 2, 915C