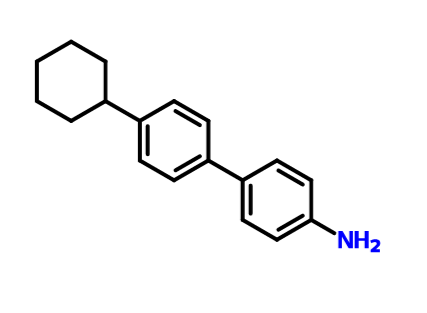
p-(4-Cyclohexylphenyl)aniline (2c)
A reported procedure was generally followed to synthesize 2c. A mixture of 4-bromocyclohexylbiphenyl (12) (1.9 g, 60 mmol, 1.0 equiv), Cu2O
(0.086 g, 0.60 mmol, 0.010 equiv), aqueous ammonia (30% solution, 8.4
mL, 120 mmol, 20 equiv), and NMP (8.4 mL, 120 mmol, 20 equiv) was
stirred at 100 °C in a sealed tube under Ar atmosphere. After 39 h, the
solution was cooled at room temperature, quenched with water, and
extracted with CH2Cl2.
The combined organic layer was washed with water and brine, dried over MgSO4, and concentrated in vacuo. The residue was purified by flash silica gel column chromatography using hexane/ethyl acetate (10/1 (v/v)) to afford 2c (0.91 g, 36 mmol, 60%) as a white powder. Mp: 101.0–101.5 °C (lit. mp 102 °C).........Basford, F. R. J. Chem. Soc. 1937, 1440– 1443, DOI: 10.1039/jr9370001440
The combined organic layer was washed with water and brine, dried over MgSO4, and concentrated in vacuo. The residue was purified by flash silica gel column chromatography using hexane/ethyl acetate (10/1 (v/v)) to afford 2c (0.91 g, 36 mmol, 60%) as a white powder. Mp: 101.0–101.5 °C (lit. mp 102 °C).........Basford, F. R. J. Chem. Soc. 1937, 1440– 1443, DOI: 10.1039/jr9370001440
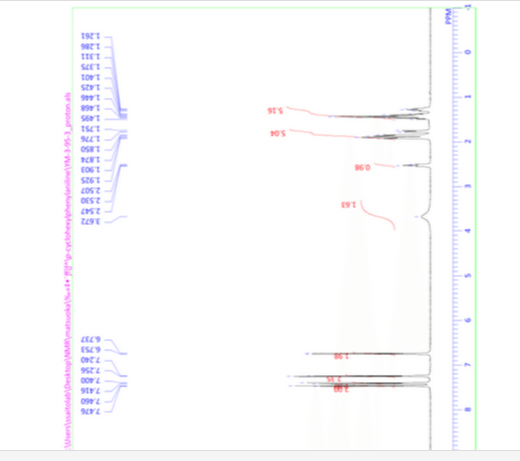
1H NMR (500 MHz, CDCl3) δ: 7.47 (d, J = 8.0 Hz, 2H), 7.41 (d, J = 8.0 Hz, 2H), 7.25 (d, J = 8.0 Hz, 2H), 6.75 (d, J = 8.0 Hz, 2H), 3.67 (br s, 2H), 2.53 (t, J = 10.0 Hz, 1H), 1.96–1.72 (m, 5H), 1.51–1.22 (m, 5H).
13C NMR (125 MHz, CDCl3) δ: 146.3, 145.7, 138.8, 131.8, 128.0, 127.3, 126.4, 115.6, 44.3, 34.7, 27.1, 26.4.
IR (ATR): 3397, 3386, 3324, 3311, 3212, 3026, 2920, 2846, 1604, 1495, 1445, 1265, 1178, 1138, 1000, 807, 692, 515, 474 cm–1.
Anal. Calcd for C18H21N: C, 86.01; H, 8.42; N, 5.57. Found: C, 86.00; H, 8.47; N, 5.58.
11H NMR BELOW
11H NMR BELOW

13C NMR
1H NMR PREDICT
13C NMR PREDICT
Synthesis and Shuttling Behavior of [2]Rotaxanes with a Pyrrole Moiety
† Department of Chemistry, Faculty of Science, Tokyo University of Science, Kagurazaka, Shinjuku, Tokyo 162-8601,Japan
‡ Faculty of Pharmaceutical Sciences, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510, Japan
§ Research Center for Medical and Dental Sciences, Tokyo Medical and Dental University, 1-5-45 Yushima, Bunkyo-ku, Tokyo 113-8510, Japan
J. Org. Chem., Article ASAP
DOI: 10.1021/acs.joc.5b02911
Publication Date (Web): March 07, 2016
Copyright © 2016 American Chemical Society
*Tel: +81-3-5228-8715. E-mail: ssaito@rs.kagu.tus.ac.jp.
Abstract
We
synthesized [2]rotaxanes with a pyrrole moiety from a [2]rotaxane with a
1,3-diynyl moiety. The conversion of the 1,3-diynyl moiety of the axle
component to the pyrrole moiety was accomplished by a Cu-mediated
cycloaddition of anilines. The cycloaddition reaction was accelerated
when the [2]rotaxane was used as the substrate. The effect of the
structure of the pyrrole moiety on the rate of the shuttling was
studied.
///////
TAKE A TOUR
Nagaon, alibaug, chaul, alibaug, MAHARASHTRA , INDIA


 Bhimeshwar temple in Nagaon
Bhimeshwar temple in Nagaon
Bhimeshwar Temple
Place of Worship
Address: Alibag-Revadanda Rd, Alibag, Maharashtra 402209









No comments:
Post a Comment