NMR Spectroscopy Theory
-
Atomic nuclei have a “spin” associated with them (i.e., they act as if
they were spinning about an axis) due to the spin associated with their
protons and neutrons.
-
Because nuclei are positively charged, their spin induces a magnetic
field. When a magnetic field is applied to atomic nuclei, the magnetic
fields of the nuclei align themselves either parallel or antiparallel to the
applied magnetic field.
-
The nuclei have a slight preference for the parallel alignment, as it
has a slightly lower energy, but nuclei can flip between the two possible
alignments.
-
When EM radiation with energy equal to the energy difference between
the two alignments is applied to the nuclei, it induces them to flip from
parallel to antiparallel alignment. Rapid flipping between alignments
occurs. The nuclei are said to be in resonance, and the energy they emit
when flipping from the high to the low energy state can be
measured.
-
The energy at which a given nucleus achieves resonance depends on its
chemical surroundings.
-
NMR spectra are taken by applying a magnetic field to as ample,
irradiating the sample with EM radiation whose energy is varied over a given
range, and measuring the energy emitted by flipping nuclei at each energy.
-
The range of radiation energies is generally chosen such
that emission from only one type of nucleus (e.g., 1H) in a
molecule is seen.
-
NMR spectroscopy does not work for nuclei that have an
even number of protons and neutrons—these nuclei have no net
spin.
-
NMR spectroscopy is most commonly done on 1H and
13C.
-
The range of radiation energies is generally chosen such
that emission from only one type of nucleus (e.g., 1H) in a
molecule is seen.
Features of an NMR Spectrum
-
NMR spectra are displayed as plots of intensity of energy emission
(due to resonance) versus the energy of the radiation applied to the sample.
Peaks in the spectrum represent resonance energies for nuclei in a
molecule.
-
Shielding: An electron cloud circulates
around each nucleus and creates a small magnetic field opposing the applied
magnetic field. The electron cloud around each atom depends on the
surrounding atoms. As a result, each nucleus experiences a slightly
different magnetic field (the sum of the applied field and the field from
the electron cloud). For this reason, the energy at which a nucleus achieves
resonance depends on its surroundings.
-
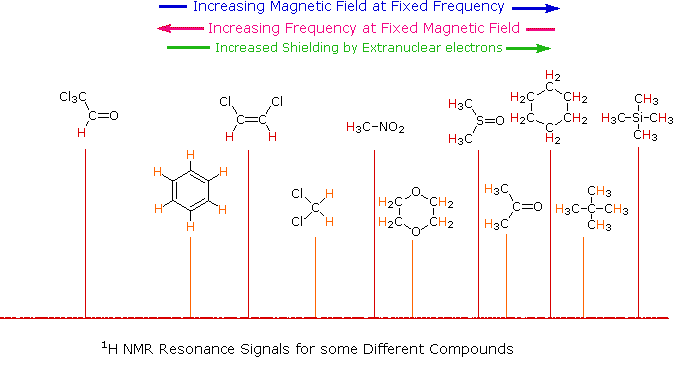
Chemical shift (δ): The resonance energy for
a given nucleus is reported in an NMR spectrum as the difference (in
parts/million) between the resonance frequency for a given proton and the
resonance frequency for protons in a reference compound, which is usually
tetramethylsilane, (CH3)4Si. Chemical shifts give information about the
atomic surroundings of a given nucleus.
-
Peak intensity: The area under a peak in an
NMR spectrum is proportional to the number of nuclei in a given chemical
environment in a molecule (e.g., if the area under a peak is two times the
area under another peak, there are twice as many nuclei responsible for the
larger peak than for the smaller one).
-
The intensity of an NMR peak gives information about the
relative number of a given type of nucleus in a
molecule.
-
The intensity of an NMR peak gives information about the
relative number of a given type of nucleus in a
molecule.
-
Spin-spin splitting: In 1H NMR, a given
hydrogen nucleus interacts with hydrogen nuclei on neighboring carbon atoms
such that the peak from that nucleus is split into multiple peaks called a
multiplet. If a hydrogen nucleus has an hydrogen nuclei on adjacent atoms,
its NMR signal is split into (n + 1) separate peaks. Relative intensities of
the peaks in a multiplet follow Pascal’s triangle.
-
Spin-spin splitting gives information about the hydrogen
atoms neighboring a given hydrogen nucleus.
-
Spin-spin splitting gives information about the hydrogen
atoms neighboring a given hydrogen nucleus.

How To Interpret An NMR Spectrum
This handout relates the basic theory of NMR
described on the theory web handout
with spectra of real molecules and how to deduce structure from the spectra.
Before reading this handout, you need to be thoroughly familiar with all
of theory concepts that were described.
1.0 The
NMR spectrum.
1.1 Because different amounts of electron density are around different non-eqivalent nuclei, the different non-equivalent nuclei in a molecule are experiencing slightly different net magnetic fields in an NMR experiment (Review Section 5.2A of the theory handout). Recall also that the difference in energy between the two allowed spin states (+1/2 and -1/2 spin states) of a spin 1/2 nucleus (like in 1H and 13C nuclei) depends on the exact magnetic field felt by the nucleus (Review Section 2.3C in the theory handout). Recall further that in the NMR experiment, when and only when nuclei are irradiated with electromagnetic radiation of energy that exactly corresponds to the energy difference between the +1/2 and -1/2 spin states, the nuclei absorb the energy and the NMR spectrometer measures this absorbance (Review section 3.1 of the theory handout). The absorbance of energy to convert a nucleus from a +1/2 to a -1/2 spin state is referred to as "resonance" of that nucleus.1.1A The key conclusion is that nuclei with different electron densities have +1/2 and -1/2 spin states that differ in energy by differing amounts, so these nuclei will absorb electromagnetic radiation of different frequencies in the NMR experiment.1.1B Nuclei surrounded by greater amounts of electron density will be more shielded from the external magnetic field, so they will absorb electromagnetic radiation of lower energy, that is, lower frequency (energy is proportional to frequency). You may want to review Section 5.2A of the theory handout again.1.1C The converse is also true, namely that nuclei surrounded by lesser amounts of electron density will be less shielded (referred to as being "deshielded") from the external magnetic field, so they will absorb electromagnetic radiation of higher energy, that is, higher frequency (energy is proportional to frequency).
1.1D The three most important factors influencing the electron density around a hydrogen nucleus are: (i) adjacent electronegative atoms remove electron density; (ii) hybridization of the attached carbon atom, increasing shielding is observed in the order sp2, sp, sp3; (iii) adjacent pi bonds are deshielding, which relates to (ii).1.2 An NMR spectrum is a plot of absorbance versus frequency.1.2A To make different spectra directly comparable, a standard is used for all NMR spectra. For 1H NMR spectra, the standard is called tetramethylsilane (TMS) and a small amount of TMS is usually added to any 1H NMR sample.1.2B Magnets of different strengths lead to absorbance of electromagnetic radiation at different frequencies for the same nucleus, meaning that if simple frequency were plotted in an NMR spectra, you could not compare spectra taken of the same sample on machines with different magnet strengths. To solve this problem, the frequency of absorption plotted on NMR spectra are corrected for the magnet strength. In addition, frequency is correlated to the reference compound TMS. The frequency at which TMS absorbs is defined as 0 frequency by convention. In the NMR spectrum, absorbance frequencies of electromagnetic radiation are plotted as chemical shift (d) listed in units called parts per million (ppm) that is defined by the following equation:2.0 The nuclear spin of hydrogen atoms creates a magnetic field that influences the chemical shift of nearby hydrogen atoms (Review Sections 5.1 and 5.2).
2.1 Nuclear spin magnetic fields will influence hydrogen atoms that are three or fewer bonds away from each other in the same molecule. Hydrogen atoms that are four or greater bonds away usually do not influence each other.
2.2 A hydrogen atom with a nuclecus in a spin state of +1/2 produces a slightly different magnetic field than a one in a –1/2 spin state.
2.3 Even in a strong magnetic field, across a population of molecules, there is only a very slight excess of nuclei in the +1/2 spin state.
2.4 Putting all of these ideas together means the following: Consider a hydrogen X adjacent (three bonds away) to another hydrogen Y in a molecule. In around half of the molecules in the NMR sample, hydrogen X feels the magnetic field from a Y with nuclear spin of +1/2. The other half feel from Y a nuclear spin of –1/2. Thus, when you look at the spectrum, there are actually two different, but closely spaced peaks as the signal for hydrogen X. This phenomenon is called “spin-spin” splitting, and the distance between the two signals for X is called the “coupling constant”, often denoted as “J”. Similarly, the signal for Y actually has two peaks because of spin-spin splitting by X.
2.5 Consider a –CH2- group adjacent to a hydrogen X. Both of the hydrogen atoms in the –CH2- are chemically equivalent and could be either in the +1/2 or –1/2 nuclear spin state. Thus, there are three situations possible: i) +1/2,+1/2; ii) +1/2,-1/2, which is the same as –1/2, +1/2 and iii) –1/2,-1/2. Thus, there are actually three different magnetic fields that are felt by X in molecules of the sample, in a 1:2:1 ratio. Thus, the signal for hydrogen X is split into three peaks in a 1:2:1 ratio.
2.6 The same holds for a –CH3 group, that will split an adjacent hydrogen signal into four peaks, with a 1:3:3:1 ratio. You should verify this for yourself by making all the possible combinations of nuclear spins for the three equivalent hydrogen atoms of a methyl group.
2.7 In the general case, N equivalent hydrogen atoms will split an adjacent signal into (N+1) peaks, with relative ratios that are predicted by Pascal’s triangle (Figure 13.16 in the book).
3.0 Following the same logic, the splitting should multiply if a single hydrogen atom is adjacent to hydrogen atoms on either side. Think about combining all the possible nuclear spin states for these nearby sets of hydrogen atoms. Thus, if you have a hydrogen atom X between one –CH2- and one –CH3 group, it should be split into an amazing (2+1) x (3 + 1) = 12 signals because there are that many different combinations of +1/2 and -1/2 spins possible.
3.1 Thus, if the coupling constants (J) from the –CH2- and –CH3 groups are significantly different from each other, then 12 peaks will be observed as the signal for hydrogen X.
3.2 However, in practice, coupling constants (J) are pretty close to the same value for almost all sets of hydrogen atoms in organic molecules, simplifying the splitting pattern, since now many of the twelve peaks will overlap with each other. What this means is that for almost all the spectra you will see, if a hydrogen X is surrounded by N hydrogen atoms, the signal for X will be split into only (N+1) peaks, no matter how those N hydrogen atoms are grouped in terms of sets of equivalent atoms. Thus, what is actually seen for the example above is that the signal for X would appear in the spectrum to be split into 2 + 3 + 1 = 6 peaks, not 12, peaks. This is the so-called “N+1” rule.
3.3 The diagram below shows these two different situations. When nuclei from hydrogen atoms Z and Y split the signal for hydrogen X with very different coupling constants (notice how the coupling constant J for the red Z hydrogen nuclei is larger than J for the blue Y hydrogen nuclei), all twelve peaks are spread out and identifiable. Below that is shown the situation in which the coupling constants are the same for nuclei of both Z and Y, so only 6 peaks are actually observed in the signal for hydrogen X due to extensive overlap. This latter case, with six peaks, is what you will almost always see in reality since coupling constants tend to be similar in organic molecules.
3.3 The above explanation of splitting can confuse students for a while. The important point is that in the example given, you see 6 different peaks in the spectrum (N+1 rule) even though there are really 12 peaks produced, it is just that several of them are on top of each other because the coupling constants are the same. For alkyl groups in organic molecules, the coupling constants are generally the same so you will almost always see the fewer peaks, corresponding to the simple N+1 rule, rather than the greater number of peaks derived from the multiplication rule.4.0 For a given signal, integrating the signal (include all splitting peaks for a given signal) gives you a relative value that is proportional to the number of equivalent hydrogen atoms that gave rise to the signal. Thus, by looking at the integration values, you can deduce how many of each type of equivalent hydrogen atoms are in the molecule. For example, a -CH3 group would have a signal that integrates to a relative value of 3 (no matter how the signal is split), and a -CH2- group would have a relative integration of 2, etc. Note that sometimes integrations are simply given as absolute numbers, and you must find the common factor to deduce how many hydrogen atoms are represented by each integration value.
3.4 The bottom line here is that by seeing how a given signal is split, you can figure out how many hydrogen atoms are adjacent on the molecule, namely the number of peaks in the signal minus 1. From this information you can piece together what a molecule looks like if you know how many atoms of each type are present (i.e. the molecular formula such as C4H10N2O). You get the molecular formula information from something called a mass spectrum, described later in the text. Molecular formulas will be provided to you in homework or test questions.
5.0 Putting it all together: How to deduce a structure from an NMR spectrum. First, you must be given the molecular formula, so you know how many of each type of atom are present. Second, count the number of different signals and their relative integrations to see how many different sets of equivalent hydrogen atoms are in a molecule, and how many of each set are present. Compare the chemical shifts of each signal to tables to identify what functional groups are present. Finally, use the signal splittings to determine which hydrogen atoms must be no more than 3 bonds away from each other.
6.0 For alkenes, the pi bond prevents bond rotation so the different hydrogen atoms on an unsymmetrical alkene are not equivalent, so they all have different signals, and splitting follows the multiplicative rule (the coupling constants are usually significantly different for geminal vs. cis. vs. trans relationships).
7.0 For hydrogens in a -CH2- group adjacent to a chiral center, the two different H atoms are no longer equivalent, because even with bond rotation, the two hydrogens are never in the same environment with respect to the groups on the adjacent chiral center. Thus, each H of -CH2- group adjacent to a chiral center usually has its own signal in the NMR spectrum.
//////////
Irbid
City in Jordan





Irbid
City in Jordan
Irbid, known in ancient times as Arabella or Arbela, is the capital and largest city of the Irbid Governorate. Wikipedia



/////////////
No comments:
Post a Comment