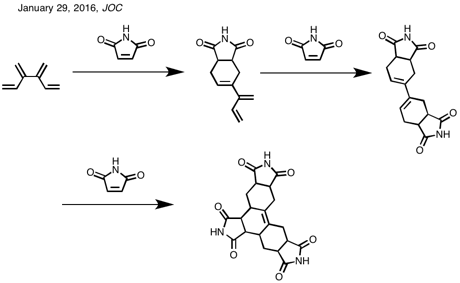
1E-Methyl[4]dendralene (10)
A freshly prepared solution of (3-methylenepenta-1,4-dien-2-yl)magnesium chloride (63) (25 mL, 0.16 M solution in THF, 4.0 mmol, 1.6 mol equiv) was added slowly into a stirred solution of ZnBr2
(0.92 g, 4.1 mmol, 1.6 mol equiv) and THF (5.0 mL) at 0 °C. Once the
addition was complete, the reaction mixture was allowed to warm to 25 °C
and stirred for a further 20 min. To this was added 1E-bromopropene (19) (0.22 mL, 2.6 mmol, 1.0 mol equiv) followed by addition of Pd(PPh3)4
(0.15 g, 0.13 mmol, 0.050 mol equiv) and the reaction mixture was
stirred at 25 °C for 16 h with the exclusion of light. The resulting
solution was poured into water (0.15 L), stirred for 15 min and
petroleum ether (30–40 °C) (0.15 L) was added. The organic phase was
separated and aqueous phase was then extracted with petroleum ether
(30–40 °C) (2 × 0.10 L). The organic phases were combined, washed with
brine, dried over MgSO4, filtered and concentrated under reduced pressure (50 mbar, 0 °C). Purification by flash column chromatography (SiO2, petroleum ether (30–40 °C)) gave the title compound 10 (0.11 g, 0.92 mmol, 35%) as a colorless oil.
Rf 0.56 (petroleum ether (30–40 °C));
1H NMR (300 MHz, CDCl3) δ 6.41 (ddd, J = 17.4, 10.5, 0.8 Hz, 1H), 6.13 (ddd, J = 15.5, 1.9, 0.7 Hz, 1H), 5.65 (dq, J = 15.6, 5.0 Hz, 1H), 5.24–5.14 (m, 2H), 5.12–5.01 (m, 3H), 4.89 (dd, J = 2.2, 0.8 Hz, 1H), 1.74 (ddt, J = 6.7, 1.5, 0.7 Hz, 3H) ppm;
13C NMR (75 MHz, CDCl3) δ 147.5 (C), 146.4 (C), 137.7 (CH), 132.1 (CH), 128.5 (CH), 117.4 (CH2), 116.5 (CH2), 115.0 (CH2), 18.2 (CH3) ppm;
IR (thin film) νmax = 3083, 2954, 2923, 2852, 1635, 1456 cm–1;
LRMS (70 eV, EI) m/z (%) 120 ([M]+•, 15%), 105 (100), 91 (44), 79 (34);
HRMS calc for C9H12 [M]+• 120.0939, found 120.0938.
http://pubs.acs.org/doi/full/10.1021/acs.joc.5b02583
Port el Kantaoui, Sousse, tunisia























//////////