General procedure for the preparation of ten newly
synthetized compounds - 2-(p-chlorophenyl)-3-methyl-4-
(p-isopropylphenyl)-1,3-thiazolium-5-(N-arylacetamide)thio
chloridrates 7a-j
Mesoionic 2-(p-chlorophenyl)-3-methyl-4-(pisopropylphenyl)-1,3-thiazolium-5-thiolate
5 (278 mmol)
was dissolved in hot ethanol and then 2-chloro-Narylacetamides
6a-j (278 mmol) were added. The system
was refluxed for 4 h and then concentrated at reduced
pressure, giving a yellow-orange solid.
R1, R2 =H, H
7a
2-(p-Chlorophenyl)-3-methyl-4-(p-isopropylphenyl)-1,3-
thiazolium-5-(N-phenylacetamide)thio chloridrate 7a
Yield: 92.13%;
m.p.: 130-132 °C; anal. calcd.: C, 60.58;
H, 4.69; N, 5.43; S, 12.44; found: C, 60.60; H, 4.70; N, 5.42;
S, 12.42;
IR (KBr) νmax / cm-1 3181 (NH), 3003 (CHAr.), 2958
(CHAlif.), 1680 (C=O), 1599, 1551, 1491 (C=C and C=N of
aromatic and heterocyclic rings), 1442 (C–N of N–CH3), 1404
(C–N), 1092 (C–Cl), 1001, 922 (CHAr.), 756 (NH), 557, 537
(C–C);
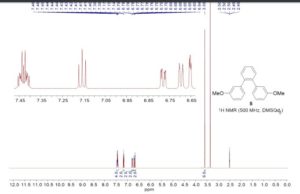
1
H NMR (200 MHz, CDCl3) delta
1.30 (d, 6H, J 6.9 Hz,
H-16, H-16’),
2.98 (sept, 1H, H-15), 3.79 (s, 2H, H-17),
3.84
(s, 3H, H-10), 7.09 (t, 2H, J 7.3 Hz, H-23),
7.28-7.34 (m,
4H, H-13, H-13’, H-21, H-25),
7.60 (d, 2H, J 8.1 Hz, H-8,
H-8’),
7.73 (d, 2H, J 7.9 Hz, H-12, H-12’),
7.86-7.93 (t,
4H, J 7.3 Hz, H-7, H-7’, H-22, H-24),
11.02 (s, 1H, H-19);
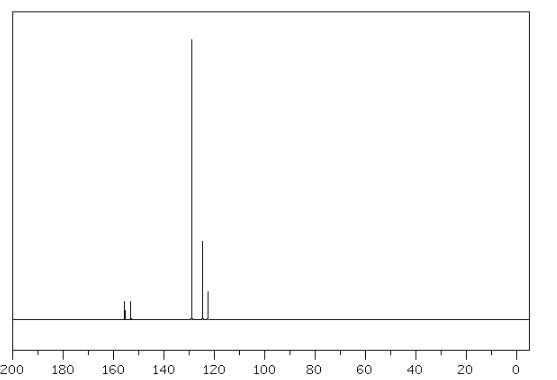
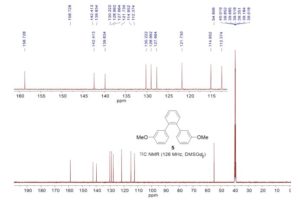
13C NMR (50 MHz, CDCl3)
delta 170.21 (C-2), 166.81 (C-18),
152.68 (C-14),
140.28 (C-4), 138.93 (C-9), 132.04 (C-20),
131.64 (C-7, C-7’), 130.89 (C-12, C-12’),
130.27 (C-8,
C-8’), 128.69 (C-22, C-24), 127.54 (C-13, C-13’),
124.09
(C-23), 123.79 (C-11), 123.59 (C-6),
120.28 (C-21, C-25),
42.24 (C-17), 40.92 (C-10), 34.21 (C-15), 23.76 (C-16, 16’).
Peixoto IN, Souza HDS, Lira BF, Silva DF, Lima EO, Barbosa-Filho JM, et al. Synthesis and Antifungal Activity AgainstCandida Strains of Mesoionic System Derived From 1,3-Thyazolium-5-thiolate. J. Braz. Chem. Soc. 2016;27(10):1807-1813
*e-mail: athayde-filho@quimica.ufpb.br
J. Braz. Chem. Soc. 2016, 27(10), 1807-1813
Isabelle N. Peixoto; Helivaldo D. S. Souza; Bruno F. Lira; Daniele F. Silva; Edeltrudes O. Lima; José M. Barbosa-Filho; Petrônio F. de Athayde-Filho
Ten new mesoionic derivatives from the 1,3-thiazolium-5-thiolate system with substituted acetamides were synthesized, had their potential as new drug evaluated in an in silico study and in their activity as antifungal against strains of Candida albicans.
Published online: February 26, 2016
http://jbcs.sbq.org.br/imagebank/pdf/151131AR.pdf

Petrônio F. de Athayde-Filho
 https://www.researchgate.net/profile/Petronio_Athayde-Filho
https://www.researchgate.net/profile/Petronio_Athayde-Filho


 //////
//////////
Dubrovnik, croatia
Dubrovnik is a city in southern Croatia fronting the Adriatic Sea. It's known for its distinctive Old Town, encircled with massive stone walls completed in the 16th century. Its well-preserved buildings range from baroque St. Blaise Church to Renaissance Sponza Palace and Gothic Rector’s Palace, now a history museum. Paved with limestone, the pedestrianized Stradun (or Placa) is lined with shops and restaurants.








...........