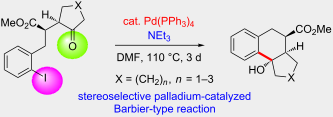
 .
.Stereoselective synthesis of tricyclic compounds by intramolecular palladium-catalyzed addition of aryl iodides to carbonyl groups
Freie Universität Berlin, Institut für Chemie und Biochemie, Takustrasse 3, D-14195 Berlin, Germany
 Corresponding author email hreissig@chemie.fu-berlin.de
Corresponding author email hreissig@chemie.fu-berlin.de
This article is part of the Thematic Series "Organometallic chemistry" and is dedicated to the memory of Professor Peter Hofmann.
Guest Editor: B. F. Straub
Beilstein J. Org. Chem. 2016, 12, 1236–1242.
doi:10.3762/bjoc.12.118
Jakub Saadi, Christoph Bentz, Kai Redies, Dieter Lentz, Reinhold Zimmer, Hans-Ulrich Reissig

Beilstein J. Org. Chem. 2016, 12, 1236–1242. published 16 Jun 2016
Abstract
Starting from γ-ketoesters with an o-iodobenzyl group we studied a palladium-catalyzed cyclization process that stereoselectively led to bi- and tricyclic compounds in moderate to excellent yields. Four X-ray crystal structure analyses unequivocally defined the structure of crucial cyclization products. The relative configuration of the precursor compounds is essentially transferred to that of the products and the formed hydroxy group in the newly generated cyclohexane ring is consistently in trans-arrangement with respect to the methoxycarbonyl group. A transition-state model is proposed to explain the observed stereochemical outcome. This palladium-catalyzed Barbier-type reaction requires a reduction of palladium(II) back to palladium(0) which is apparently achieved by the present triethylamine.
For our systematic studies on samarium diiodide promoted cyclizations leading to benzannulated medium-sized rings [1-4] we required starting materials such as alkenyl-substituted compounds B (Scheme 1). Obvious precursors for B are aryl iodides A that smoothly undergo palladium-catalyzed coupling reactions to provide the desired products. However, in one case [A: R1–R2 = (CH2)4] typical Heck reaction conditions employing styrene as olefin component not only led to the desired styrene derivative B but mainly to the cyclized product C. If the reaction was performed without the olefin it provided only the tertiary alcohol C in reasonable yield [5]. Similar C–C bond forming reactions of aryl halides that involve an insertion of the intermediate aryl palladium species into a carbonyl group are relatively rare (see discussion below). Therefore this serendipitous discovery led us to investigate the reaction in more detail.
Scheme 1: Planned Heck reaction of A to compound B and serendipitous discovery of the palladium-catalyzed cyclization to products C.
Conclusion
We have found new examples of intramolecular palladium-catalyzed nucleophilic additions of aryl iodides to alkyl ketones. These additions proceed in the presence of only 2–5 mol % Pd(PPh3)4 and afford bi- and tricyclic compounds with excellent stereoselectivity and in moderate to very good efficacy. The low mass balance observed in several cases may be due to subsequent reactions such as simple de-iodination of the precursor compounds or elimination of water in the products. However, in general none of these byproducts has been isolated. For compound 2 the bulky isopropyl group slows down the addition to the carbonyl group and an enolate arylation was observed instead as major reaction pathway. Although the scope of the discovered aryl iodide addition to carbonyl groups may be limited it is attractive since only low catalyst loadings are required and interesting products are formed with high stereoselectivity.
Methyl (2RS,4RS)-4-hydroxy-4-isopropyl-1,2,3,4-tetrahydronaphthalene-2-carboxylate
Methyl (2RS,4RS)-4-hydroxy-4-isopropyl-1,2,3,4-tetrahydronaphthalene-2-carboxylate (8):
According to the GP1: compound 2 (200 mg, 0.535 mmol), Pd(PPh3)4 (30 mg, 27 µmol), NEt3 (18 mg,
1.78 mmol), DMF (4 mL), 90 °C, 3 d. Column chromatography (silica gel, hexanes/ethyl acetate 4:1 to
1:1) provided 14 mg (11%) of 8, 33 mg (25%) of 9 and 82 mg (62%) of 10 as colorless oils.
1H NMR (CDCl3, 500 MHz): δ = 0.60, 1.13 (2 d, J = 6.9 Hz, 2 × 3 H, CHMe2), 1.84 (dd, J = 13.9, 12.3
Hz, 1 H, 3-H), 1.95 (s, 1 H, OH), 2.17 (ddd, J = 13.9, 2.7, 2.4 Hz, 1 H, 3-H), 2.47 (sept, J = 6.9 Hz, 1 H,
CHMe2), 2.81 (dd, J = 14.5, 12.7 Hz, 1 H, 1-H), 2.88 (dddd, J = 12.7, 12.3, 2.7, 2.6 Hz, 1 H, 2-H), 2.98
(ddd, J = 14.5, 2.6, 2.4 Hz, 1 H, 1-H), 3.73 (s, 3 H, CO2Me), 7.10-7.25, 7.48-7.49 (2 m, 3 H, 1 H, Ar)
ppm. MS (EI = 70 eV): m/z (%) = 248 (1) [M]+ , 217 (4), 242 (6), 205 (100), 173 (32), 145 (62).
/////////1,2-addition, aryl iodides, ketones, nucleophilic addition, palladium catalysis,


/////////




![[1860-5397-12-118-i1]](http://www.beilstein-journals.org/bjoc/content/inline/1860-5397-12-118-i1.png?max-width=550&background=EEEEEE)