
Example 7 - Preparation Of Compound 7 (4-(3-(methylsulfinyl)phenyl)-l-propyl-l,2,3,6- tetrahydropyridine)

4-(3-(methylsulfinyl)phenyl)-1-propyl- 1 ,2,3,6-tetrahydropyridin-1-ium chloride Sulfuric acid (42.23g, 0.43 lmol, leq) was added to a mixture of 4-hydroxy-4-(3- (methylsulfonyl)phenyl)-l-propylpiperidin-l-ium chloride (130g, 0.431 mo, leq) and toluene (650mL) at room temperature. The resulting two-phase solution was refluxed for lhour and HPLC showed that the product reached 95% area. The reaction mixture was cooled down to 20°C and the toluene phase was decanted to give viscous residue that was diluted with water (600mL) and neutralized with 2N NaOH to pH~4.2. Hydrogen peroxide (50%, 32.21g, 0.474mol, l.leq) was added dropwise to the water phase and the mixture was stirred at 60°C for lh after which the product reached 96% area (HPLC).
Toluene (600mL) was added to the reaction mixture and made basic first with 25% NaOH (60g) and finally with 10% NaOH up to pH 12. The phases were separated and the water phase was re-extracted with toluene (2xl00mL). The combined toluene phases were washed with 5% sodium sulfite (150mL), brine (150mL) and water (150mL). The toluene phase was then concentrated under vacuum on a rotavapor to give 111.3g oil (HPLC area: 96.6%). Methanol (50mL) was added to the residue and it was filtered and cooled down on ice batch. Dry HC1 in ethyl acetate was added up to pH 1-2 (120mL) and lOOmL of ethyl ether were added to give two phases mixture. The mixture was seeded with the product and precipitation started. The reaction mixture was stirred on ice bath (2-5°C) for additional lh, filtered and washed with 1/3 ethyl acetate/ether mixture (lOOmL) to give 140g of very hygroscopic light yellow solid that was dried on a rotavapor for 2h and stored under nitrogen in deep freeze. The dry 4-(3-(methylsulfinyl)phenyl)-l-propyl-l,2,3,6-tetrahydropyridine-HCl is slightly yellowish solid (94.1g, 79% yield, HPLC (254nm): 96.3% area, 1H-NMR assay: 97.5%).
NMR Identity Analysis of Compound 7
Compound 7: 

The following data in Tables 14 and 15 was determined using a sample of Compound 7, a solvent of CDCb, and the instruments were a Bruker AMX500 and Avance III 800 MHz instrument.
Table 14: Assignment of ¾ NMR"

"Spectra is calibrated by the solvent residual peak (2.5 ppm).
bafter addition of small amount of CeDe

a Spectra is calibrated by a solvent peak (77.0 ppm)


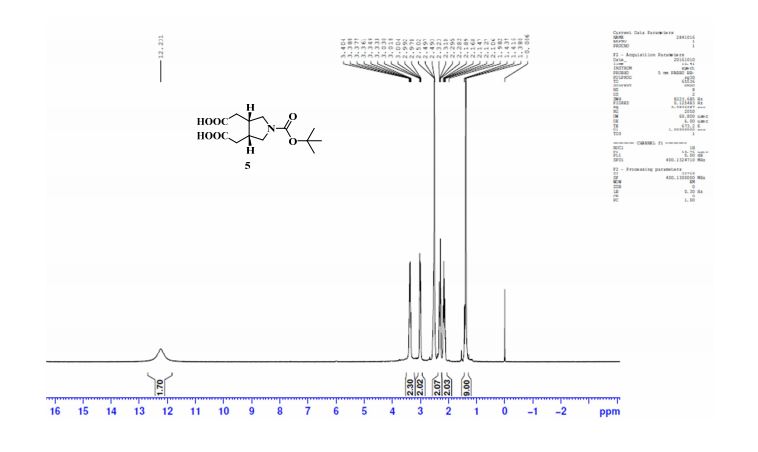
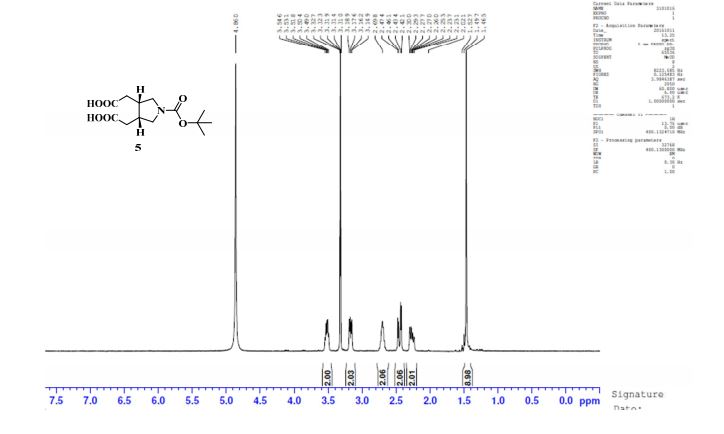
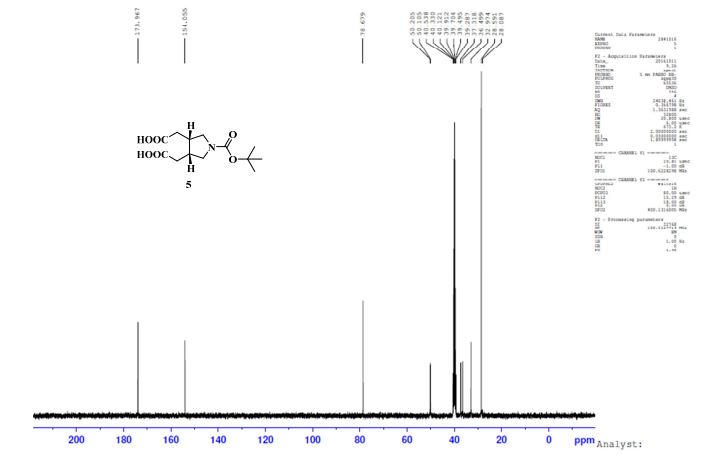
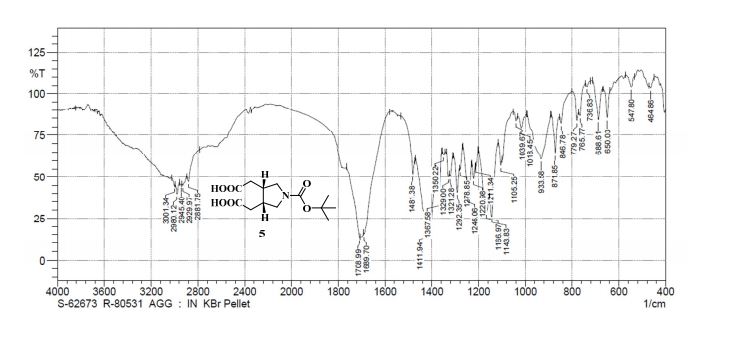





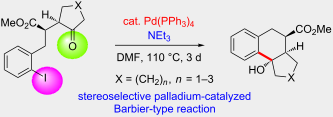 .
. Corresponding author email hreissig@chemie.fu-berlin.de
Corresponding author email hreissig@chemie.fu-berlin.de![[1860-5397-12-118-i1]](http://www.beilstein-journals.org/bjoc/content/inline/1860-5397-12-118-i1.png?max-width=550&background=EEEEEE)


