1 H NMR

1 it can be seen that from the H NMR, in the compound in the number of protons (integral value) and is dependent on the electronic state chemical shift (ppm) and, dependent on the number of adjacent proton coupling there is. To make a structural analysis, first, one look at the H NMR, where the chemical shift of some, there are any number of protons, and then determines whether you are what coupling (binding constant). Introduction coupling for. Proton of the molecule, as shown above

geminal and vicinal when magnetically non-equivalent proton is present in the position, it makes the coupling (division). Proton each other that the coupling together, the samecoupling constant ( J value) has a (crack width), sp 3 in the coupling of the geminal proton each other is attached to the hybridized carbon is 10 Hz position, vicinal proton each other For, it has a value that depends on the dihedral angle of the two protons as shown in the figure below. When the dihedral angle is close to 0 and 180 ° takes a large value, you can see that taking a small value when it is close to 90 °.

Here an example of protons that are coupled.

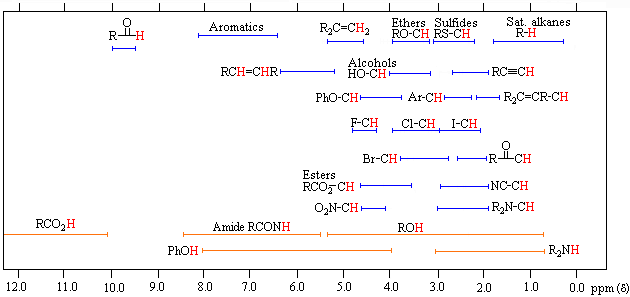
The figure above shows the picture of a case where there proton is a total of two geminal and vicinal proton coupling. ①: a state in which nothing has been coupling. ( s: singlet ) ②: state in which the coupling between one proton coupling constant a. ( D: doublet ) ③: ② state proton is another proton coupling in a more binding constant a of. ( dd: double doublet ) or ① state proton is coupled magnetically equivalent to two protons. ( t: Triplet ) ④: ② state proton is another protons and coupling in the near value (b) to further coupling constant a of. ( dd ) (slightly overlapping the center of the mountain, become larger than the outside of the mountain.) ⑤: ② state proton is another protons and coupling with significantly different value (c) the further coupling constant a of. ( dd ) (the height of the peak of 1: 1: 1: to 1) above diagram depicts the picture of a case where there proton is coupled with a total of three of geminal and vicinal proton. ⑥: ③ state proton is another proton coupling in a more binding constant a of. ( ddd: double double doublet ) or ① state proton is coupled magnetically equivalent to three protons. ( Q: Quartet ) (height of the peak is approximately 1: 3: 3: become 1)⑦: ③ state proton is another proton coupling further in coupling constant a smaller value (d) of. ( ddd ) ⑧: ③ state proton is another protons and coupling with a very large value (e) than further coupling constant a of. ( ddd ) then chemical shift for. The above figure, proton in a variety of environments can be taken, it shows the approximate chemical shift values. Proton bound to a benzene ring or a double bond takes a value of 6 ~ 8 ppm, methine present in the oxygen base of the ester, methylene, you can see that it is 4 ~ 5 ppm. Moreover, methine nitrogen atom is bonded to an oxygen atom, a methylene is 2.6 ~ 4.1 ppm, a hydrocarbon that does not bind to their heteroatom takes the value of 0.8 ~ 2.2 ppm. In addition, the integral value for (number of protons).



1 it can be seen that from the H NMR, in the compound in the number of protons (integral value) and is dependent on the electronic state chemical shift (ppm) and, dependent on the number of adjacent proton coupling there is. To make a structural analysis, first, one look at the H NMR, where the chemical shift of some, there are any number of protons, and then determines whether you are what coupling (binding constant). Introduction coupling for. Proton of the molecule, as shown above
geminal and vicinal when magnetically non-equivalent proton is present in the position, it makes the coupling (division). Proton each other that the coupling together, the samecoupling constant ( J value) has a (crack width), sp 3 in the coupling of the geminal proton each other is attached to the hybridized carbon is 10 Hz position, vicinal proton each other For, it has a value that depends on the dihedral angle of the two protons as shown in the figure below. When the dihedral angle is close to 0 and 180 ° takes a large value, you can see that taking a small value when it is close to 90 °.
Here an example of protons that are coupled.
The figure above shows the picture of a case where there proton is a total of two geminal and vicinal proton coupling. ①: a state in which nothing has been coupling. ( s: singlet ) ②: state in which the coupling between one proton coupling constant a. ( D: doublet ) ③: ② state proton is another proton coupling in a more binding constant a of. ( dd: double doublet ) or ① state proton is coupled magnetically equivalent to two protons. ( t: Triplet ) ④: ② state proton is another protons and coupling in the near value (b) to further coupling constant a of. ( dd ) (slightly overlapping the center of the mountain, become larger than the outside of the mountain.) ⑤: ② state proton is another protons and coupling with significantly different value (c) the further coupling constant a of. ( dd ) (the height of the peak of 1: 1: 1: to 1) above diagram depicts the picture of a case where there proton is coupled with a total of three of geminal and vicinal proton. ⑥: ③ state proton is another proton coupling in a more binding constant a of. ( ddd: double double doublet ) or ① state proton is coupled magnetically equivalent to three protons. ( Q: Quartet ) (height of the peak is approximately 1: 3: 3: become 1)⑦: ③ state proton is another proton coupling further in coupling constant a smaller value (d) of. ( ddd ) ⑧: ③ state proton is another protons and coupling with a very large value (e) than further coupling constant a of. ( ddd ) then chemical shift for. The above figure, proton in a variety of environments can be taken, it shows the approximate chemical shift values. Proton bound to a benzene ring or a double bond takes a value of 6 ~ 8 ppm, methine present in the oxygen base of the ester, methylene, you can see that it is 4 ~ 5 ppm. Moreover, methine nitrogen atom is bonded to an oxygen atom, a methylene is 2.6 ~ 4.1 ppm, a hydrocarbon that does not bind to their heteroatom takes the value of 0.8 ~ 2.2 ppm. In addition, the integral value for (number of protons).
In the figure (1) to (5) all is 1 pieces of protons, and chemical shift, it is shown the case where the coupling is different.
As you can see, even in the same one piece of the proton, we can see that the height of the peak by way of coupling is different.
Basically, the more to increase the coupling, but peak height tend to be low, the integral value of each proton (area) does not change.
If the peak height differ greatly even though it has already been a coupling similar, consider the number of protons is different. Well, there is a method of determining the chemical shift, which is one of the center of gravity of the proton is may be obtained a. Singlet: The value of the apex of the peak, doublet: the average value of the vertices of the two peaks triplet: The value of the apex of the peak of the middle quartet: the average value of the apex of the peak of the both ends or ... asked how would each person? By the way, the case of the above figure, (1) In the case of: There is one proton to 2.01 ppm, coupling s (singlet). (2) In the case of: located in one of the protons is 5.42 ppm, coupling d (doublet), coupling constant (J) is (5.423-5.410) × 800 = 10.4 from 10.4 Hz. (3) In the case of: There is one proton to 3.40, coupling dd (double doublet), coupling constant (J) is (3.409-3.393) ÷ 2 × 800 = 6.4 than 6.4, 6.4 Hz. (4) In the case of: Yes to the 6.32 ppm 1 single proton, the coupling dd (double doublet), coupling constant (J) is (6.339-6.318) × 800 = 16.8 than one is 16.8 Hz. The other, (6.339-6.330) × 800 = 7.2 than 7.2 Hz. In the case of (5): There is one proton to 1.55 ppm, coupling ddd (double double doublet), coupling constant (J) is (1.563-1.551) ÷ 2 × 800 = 4.8 than 4.8, 4.8 Hz. The other, (1.563-1.560) × 800 = 2.4 than 2.4 Hz. And it will decipher. Chemical shifts, rounded to two decimal places, on the other hand, the coupling constant is good to round off the second decimal place. ppm of the display 1 when calculating the binding constants from the H NMR should multiplied by the resonant frequency of the device to the difference between the peak apex. In the case of the figure above, it has 800 is used for the calculation formula because using NMR of 800 MHz. Moreover, when put together as data, if the (1), 2.01 (1H, s) In the case of (5), 1.55 (1H, ddd, 4.8, 4.8, 2.4) and will be referred.
As you can see, even in the same one piece of the proton, we can see that the height of the peak by way of coupling is different.
Basically, the more to increase the coupling, but peak height tend to be low, the integral value of each proton (area) does not change.
If the peak height differ greatly even though it has already been a coupling similar, consider the number of protons is different. Well, there is a method of determining the chemical shift, which is one of the center of gravity of the proton is may be obtained a. Singlet: The value of the apex of the peak, doublet: the average value of the vertices of the two peaks triplet: The value of the apex of the peak of the middle quartet: the average value of the apex of the peak of the both ends or ... asked how would each person? By the way, the case of the above figure, (1) In the case of: There is one proton to 2.01 ppm, coupling s (singlet). (2) In the case of: located in one of the protons is 5.42 ppm, coupling d (doublet), coupling constant (J) is (5.423-5.410) × 800 = 10.4 from 10.4 Hz. (3) In the case of: There is one proton to 3.40, coupling dd (double doublet), coupling constant (J) is (3.409-3.393) ÷ 2 × 800 = 6.4 than 6.4, 6.4 Hz. (4) In the case of: Yes to the 6.32 ppm 1 single proton, the coupling dd (double doublet), coupling constant (J) is (6.339-6.318) × 800 = 16.8 than one is 16.8 Hz. The other, (6.339-6.330) × 800 = 7.2 than 7.2 Hz. In the case of (5): There is one proton to 1.55 ppm, coupling ddd (double double doublet), coupling constant (J) is (1.563-1.551) ÷ 2 × 800 = 4.8 than 4.8, 4.8 Hz. The other, (1.563-1.560) × 800 = 2.4 than 2.4 Hz. And it will decipher. Chemical shifts, rounded to two decimal places, on the other hand, the coupling constant is good to round off the second decimal place. ppm of the display 1 when calculating the binding constants from the H NMR should multiplied by the resonant frequency of the device to the difference between the peak apex. In the case of the figure above, it has 800 is used for the calculation formula because using NMR of 800 MHz. Moreover, when put together as data, if the (1), 2.01 (1H, s) In the case of (5), 1.55 (1H, ddd, 4.8, 4.8, 2.4) and will be referred.
13 C NMR
13 it can be seen from C NMR is the number of carbon atoms in the compound is dependent on the electronic state chemical shift (ppm) it is.
All of the signal is a singlet, you do not need to seek the binding constant. If a large number of protons attached to carbon atoms, or an easily measured, there is a tendency that the height of the signal is high.Chemical shift is good when rounded to 2 decimal places.
All of the signal is a singlet, you do not need to seek the binding constant. If a large number of protons attached to carbon atoms, or an easily measured, there is a tendency that the height of the signal is high.Chemical shift is good when rounded to 2 decimal places.
From HMQC, C at a distance of Ha and 1 Bond 1 it will give correlation with.
Correlation shown in red, the carbon atoms of 61.9 ppm, represents that the bond is 2.74 and 2.84 ppm proton.
Moreover, the correlation shown in blue, to a carbon atom of 41.3 ppm, represents that the bond is 2.25 and 2.38 ppm proton.
Area shown in green, will not overlap a lot of correlation. Analysis give up please. It may be able to be analyzed by other means.
Moreover, the correlation shown in blue, to a carbon atom of 41.3 ppm, represents that the bond is 2.25 and 2.38 ppm proton.
Area shown in green, will not overlap a lot of correlation. Analysis give up please. It may be able to be analyzed by other means.
Ha If you pay attention to,
1 H 1 from the H COSY, Ha and Hb, c, obtained correlation with d.
(Correlation between protons at a distance of 2, 3 bond from Ha) chart While are separated by a triangular red and blue, two regions are symmetrical, that is sufficient to analyze all either one of a pair It will be. For example, correlation and shown in light blue, both the correlation shown in purple, because it represents the correlation of 2.25 ppm and 2.38 ppm proton, and decrypt both, you will duplication of effort. Correlation shown in yellow shows the correlation of 2.56 ppm protons and 1.92 ppm proton. In addition, from the correlation shown in green, of 2.56 ppm proton further shows that there is a correlation with the 2.68 ppm proton.

(Correlation between protons at a distance of 2, 3 bond from Ha) chart While are separated by a triangular red and blue, two regions are symmetrical, that is sufficient to analyze all either one of a pair It will be. For example, correlation and shown in light blue, both the correlation shown in purple, because it represents the correlation of 2.25 ppm and 2.38 ppm proton, and decrypt both, you will duplication of effort. Correlation shown in yellow shows the correlation of 2.56 ppm protons and 1.92 ppm proton. In addition, from the correlation shown in green, of 2.56 ppm proton further shows that there is a correlation with the 2.68 ppm proton.
From HMBC, C is a distance from Ha to 3 Bond 1 , C 2 , C 3 , C 5 , C 6 and the correlation is obtained with.
Correlation shown in red, shows the carbon correlation of 2.56 ppm protons and 39.9 ppm. In addition, the correlation shown in blue, of 2.56 ppm proton, shows that there is a correlation with carbon of 56.1 ppm. Green, correlations shown in purple represents that there is a correlation of two protons 3.00 and 3.06 ppm for the carbon of 52.8 ppm. On the other hand, the correlation shown in yellow shows the correlation between 10.0 ppm of carbon, but also try the attribution of the protons by tracing the correlation, I can not find proton. This correlation is observed in HMQC (1bond), in HMBC, because there is a feature that causes division. The 10.0 ppm proton location where there is a correlation with the carbon of the case will be 0.97 ppm is a midpoint of 0.85 and 1.08 ppm. Such a correlation, when, let's note Ayamarase the analysis. NOESY
From NOESY, Hb in the Ha and spatially close distance, c, d, e, I correlation with i is obtained. NOESY is mainly used when assigning a relative placement. COSY Similarly, blue and red of the area, but has become a nearly symmetrical, etc. If the correlation is poor, who both were firmly analysis would be better. Well, in the NOESY, as observed even COSY, associative even seen a correlation between close proton (2,3bond) It is not a wonder. Therefore, it can be said from the charts NOESY, information excluding the correlation seen in the chart of COSY is especially useful information. Yellow, green, correlations shown in purple shows a NOESY correlation in not observed COSY. With that collect such information in detail, you might be able to infer the relative placement.

Other
Or let's say even with the extended version of the COSY.
From HOHAHA, Ha and Hb, c, d, e, f, g, h, I correlation is obtained with j.
(If there are quaternary carbon in the carbon chain, the correlation between the above in the proton can not be obtained basically .Ha and Hk, l, m, n, o, etc..)
From HOHAHA, Ha and Hb, c, d, e, f, g, h, I correlation is obtained with j.
(If there are quaternary carbon in the carbon chain, the correlation between the above in the proton can not be obtained basically .Ha and Hk, l, m, n, o, etc..)