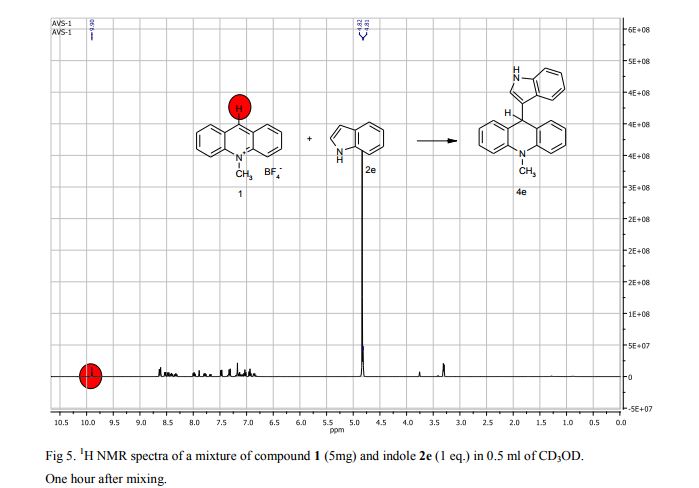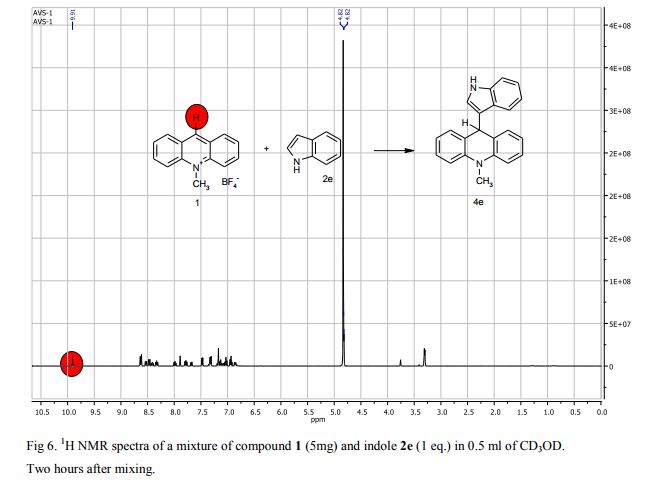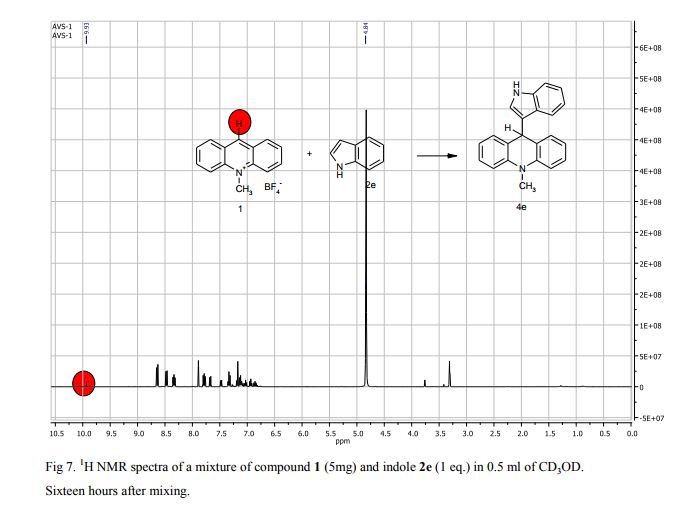
Metal-free oxidative cyclization of 2-aminobenzothiazoles and cyclic ketones enabled by the combination of elemental sulfur and oxygen
Green Chem., 2017, Advance Article
DOI: 10.1039/C7GC02014G, Communication
DOI: 10.1039/C7GC02014G, Communication
Yanjun Xie, Xiangui Chen, Zhen Wang, Huawen Huang, Bing Yi, Guo-Jun Deng
Aerobic cyclization of 2-aminobenzothiazoles and cyclic ketones enabled by the combination of elemental sulfur and oxygen under metal-free conditions.
Aerobic cyclization of 2-aminobenzothiazoles and cyclic ketones enabled by the combination of elemental sulfur and oxygen under metal-free conditions.
Metal-free oxidative cyclization of 2-aminobenzothiazoles and cyclic ketones enabled by the combination of elemental sulfur and oxygen
Abstract
Metal-free oxidative cyclization for the one-pot synthesis of benzo[d]imidazo[2,1-b]thiazoles from 2-aminobenzothiazoles and cyclic ketones is described. Elemental sulfur combined with molecular oxygen as the benign co-oxidant was found to be unique and highly effective to promote this transformation without the aid of any metal salts. Various cyclic ketones smoothly reacted with 2-aminobenzothiazoles to give functional benzo[d]imidazo[2,1-b]thiazoles in good to very high yields, which thereby demonstrated the synthetic convergence of this methodology.
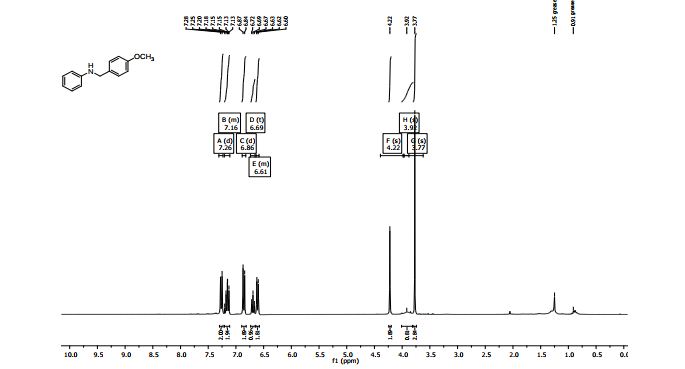
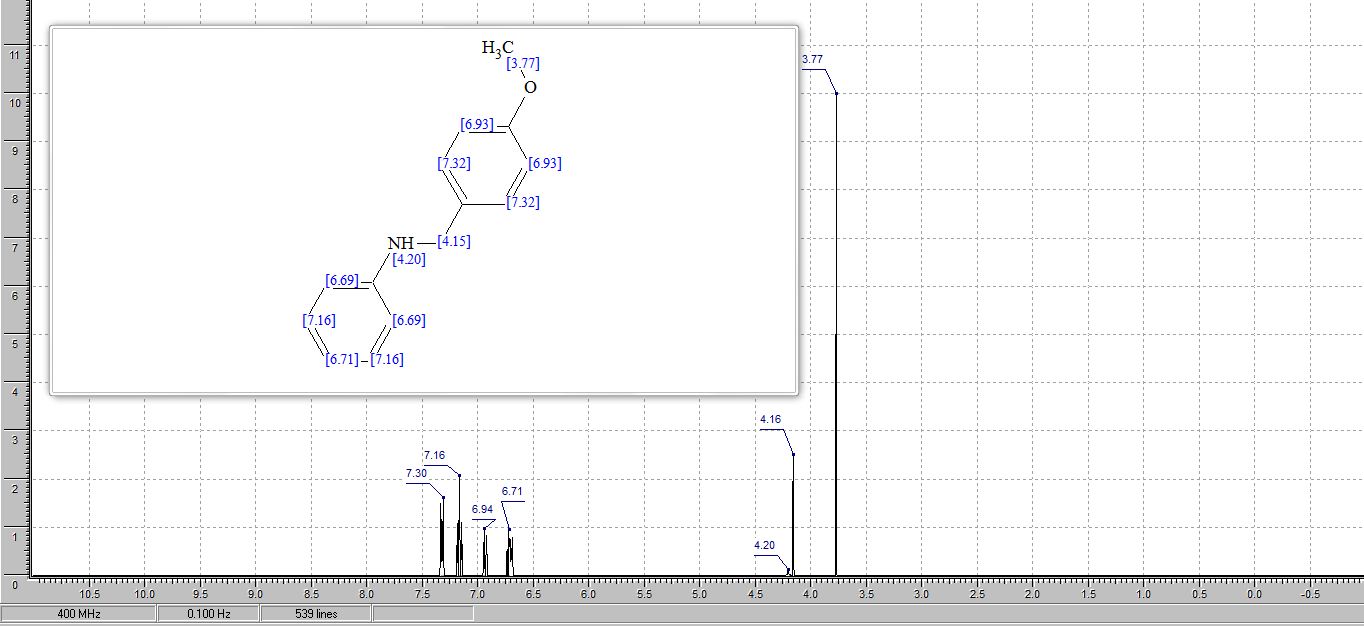
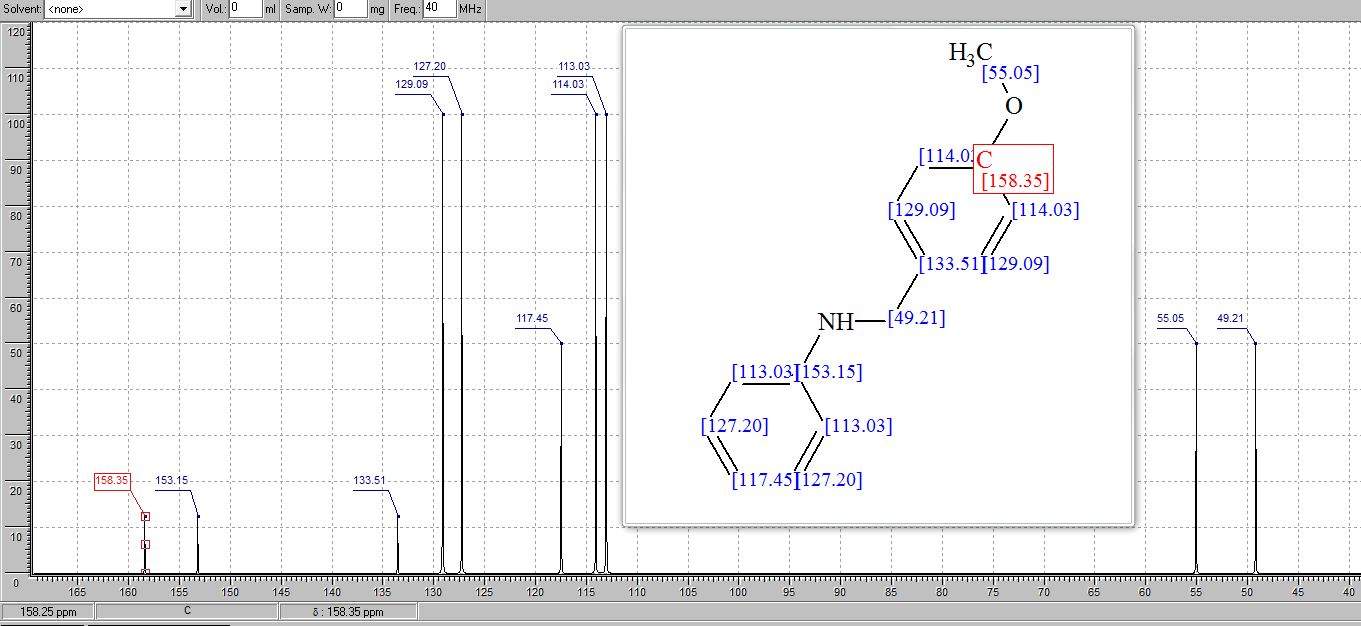
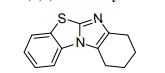
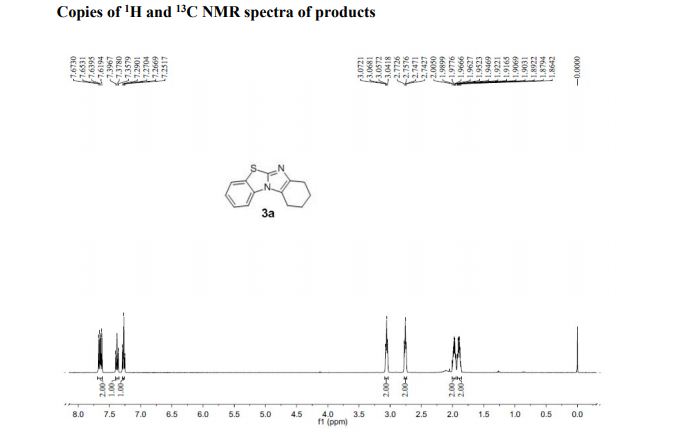
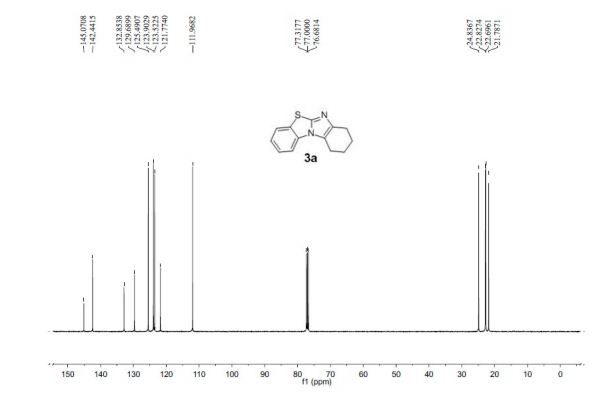
7,8,9,10-Tetrahydrobenzo[d]benzo[4,5]imidazo[2,1-b]thiazole (3a)
White solid; yield: 39.2 mg (86%), mp 140-142 °C.
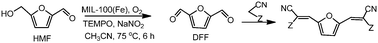
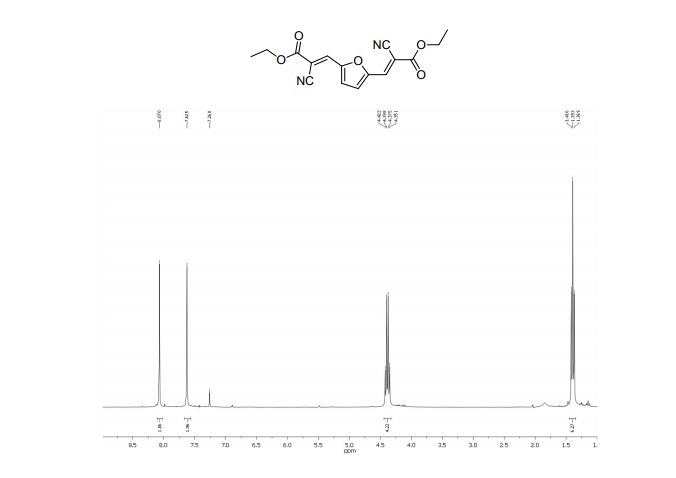
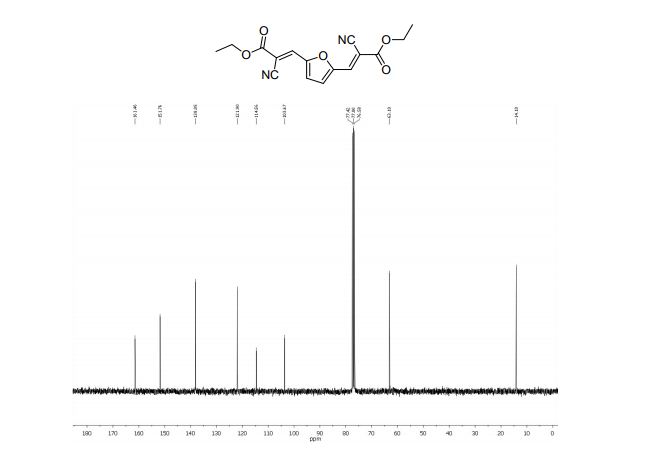
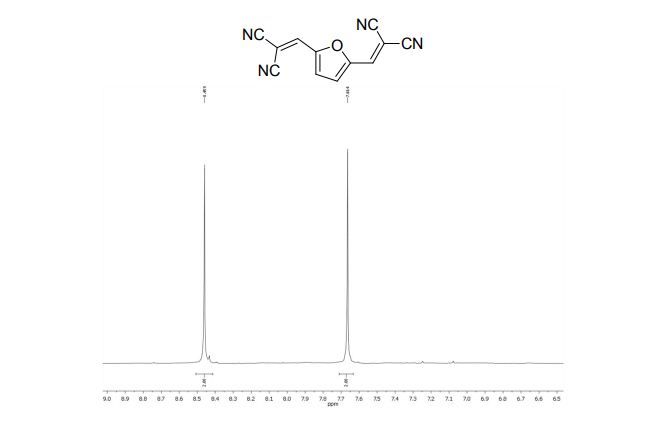
1H NMR (400 MHz, CDCl3, ppm) δ 7.67-7.62 (m, 2H), 7.38 (t, J = 7.76 Hz, 1H), 7.27 (t, J = 7.68 Hz, 1H), 3.07-3.04 (m, 2H), 2.77-2.74 (m, 2H), 2.00-1.95 (m, 2H), 1.92-1.86 (m, 2H);
13C NMR (100 MHz, CDCl3, ppm) δ 145.1, 142.4, 132.9, 129.7, 125.5, 123.9, 123.5, 121.8, 111.9, 24.8, 22.8, 22.7, 21.8;
MS (EI) m/z (%) 228, 200 (100), 160, 108, 51;
HRMS calcd. for: C13H13N2S + (M+H)+ 229.07940, found 229.07941.
PREDICT
cas 325766-28-7
C13 H12 N2 S, 228.31, Benzimidazo[2,1-b]benzothiazole, 7,8,9,10-tetrahydro-
///////////////
C1CCCc2c1nc3sc4ccccc4n23
 Open Access
Open Access