Riluzole (Rilutek) is a drug used to treat amyotrophic lateral sclerosis and is marketed by Sanofi-Aventis. It delays the onset ofventilator-dependence or tracheostomy in selected patients and may increase survival by approximately two to three months.[2]
NMR.........http://file.selleckchem.com/downloads/nmr/S161403-Riluzole-NMR-Selleck.pdf

NMR.........http://file.selleckchem.com/downloads/nmr/S161403-Riluzole-NMR-Selleck.pdf
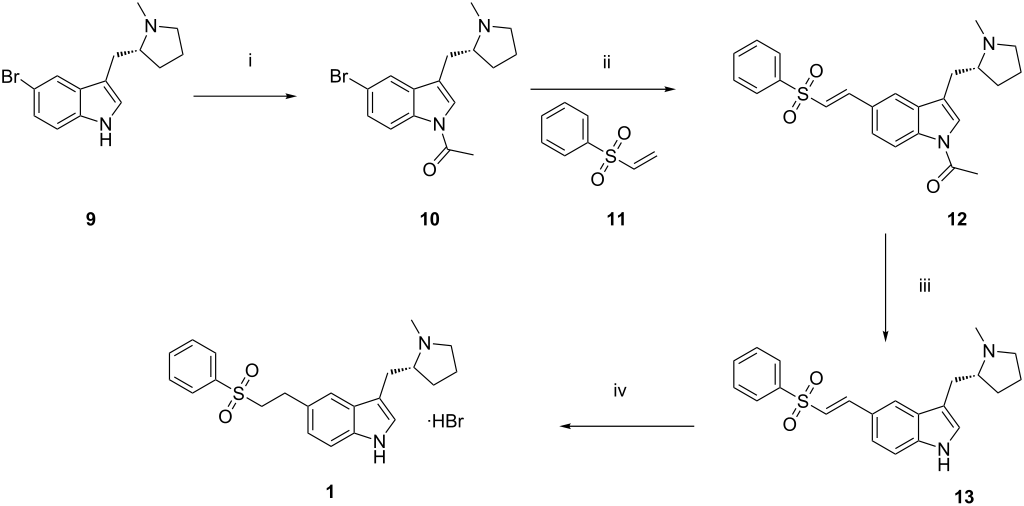
RILUTEK® (riluzole) is a member of the benzothiazole class. Chemically, riluzole is 2-amino-6-(trifluoromethoxy)benzothiazole. Its molecular formula is C8H5F3N2OS and its molecular weight is 234.2. Its structural formula is as follows:
Riluzole is a white to slightly yellow powder that is very soluble in dimethylformamide, dimethylsulfoxide and methanol, freely soluble in dichloromethane, sparingly soluble in 0.1 N HCl and very slightly soluble in water and in 0.1 N NaOH. RILUTEK (riluzole) is available as a capsule-shaped, white, film-coated tablet for oral administration containing 50 mg of riluzole. Each tablet is engraved with “RPR 202” on one side.

Mechanism
Riluzole preferentially blocks TTX-sensitive sodium channels, which are associated with damaged neurons.[12][13] Riluzole has also been reported to directly inhibit the kainateand NMDA receptors.[14] However, the action of riluzole on glutamate receptors has been controversial, as no binding of the drug to any known sites has been shown for them.[15][16] In addition, as its antiglutamatergic action is still detectable in the presence of sodium channel blockers, it is also uncertain whether or not it acts via this way. Rather, its ability to stimulate glutamate uptake seems to mediate many of its effects.[17][18] In addition to its role in accelerating glutamate clearance from the synapse, Riluzole may also prevent glutamate release from presynaptic terminals.[19] These effects combined could significantly reduce glutamate signaling and cause indirect antagonism without acting at glutamate receptors themselves.
Synthesis

References[edit]
- ^ a b c d e f g h i "PRODUCT INFORMATION RILUTEK® (riluzole) Tablets" (PDF). TGA eBusiness Services. sanofi-aventis australia pty ltd. 6 January 2009. Retrieved 18 February2014.
- ^ a b Miller, RG; Mitchell, JD; Moore, DH (14 March 2012). "Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND)." (PDF). The Cochrane Database of Systematic Reviews 3: CD001447. doi:10.1002/14651858.CD001447.pub3.PMID 22419278.
- ^ van Kan, HJ; Groeneveld, GJ; Kalmijn, S; Spieksma, M; van den Berg, LH; Guchelaar, HJ (March 2005). "Association between CYP1A2 activity and riluzole clearance in patients with amyotrophic lateral sclerosis." (PDF). British Journal of Clinical Pharmacology 59(3): 310–3. doi:10.1111/j.1365-2125.2004.02233.x. PMC 1884790. PMID 15752377.
- ^ Review of the Use of the Glutamate Antagonist Riluzole in Psychiatric Disorders and a Description of Recent Use in Childhood Obsessive-Compulsive Disorder. J Child Adolesc Psychopharmacol. 2010 August; 20(4): 309–315.
- ^ Zarate CA, Jr; Payne, JL; Quiroz, J; Sporn, J; Denicoff, KK; Luckenbaugh, D; Charney, DS; Manji, HK (January 2004). "An open-label trial of riluzole in patients with treatment-resistant major depression.". The American Journal of Psychiatry 161 (1): 171–4.doi:10.1176/appi.ajp.161.1.171. PMID 14702270.
- ^ Coric, V; Taskiran, S; Pittenger, C; Wasylink, S; Mathalon, DH; Valentine, G; Saksa, J; Wu, YT; Gueorguieva, R; Sanacora, G; Malison, RT; Krystal, JH (1 September 2005). "Riluzole augmentation in treatment-resistant obsessive-compulsive disorder: an open-label trial.". Biological Psychiatry 58 (5): 424–8. doi:10.1016/j.biopsych.2005.04.043.PMID 15993857.
- ^ Mathew, SJ; Amiel, JM; Coplan, JD; Fitterling, HA; Sackeim, HA; Gorman, JM (December 2005). "Open-label trial of riluzole in generalized anxiety disorder.". The American Journal of Psychiatry 162 (12): 2379–81. doi:10.1176/appi.ajp.162.12.2379.PMID 16330605.
- ^ "Glutamatergic regulation prevents hippocampal-dependent age-related cognitive decline through dendritic spine clustering". Proceedings of the National Academy of Sciences of the United States of America. Retrieved 18 January 2015.
- ^ "Glutamatergic Dysfunction in Cognitive Aging: Riluzole in Mild Alzheimers Disease".rucares.org. Retrieved 12 March 2015.
- ^ "Rilutek (riluzole) dosing, indications, interactions, adverse effects, and more".Medscape Reference. WebMD. Retrieved 18 February 2014.
- ^ a b c Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- ^ Song, JH; Huang, CS; Nagata, K; Yeh, JZ; Narahashi, T (August 1997). "Differential action of riluzole on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels."(PDF). The Journal of Pharmacology and Experimental Therapeutics 282 (2): 707–14.PMID 9262334.
- ^ Bellingham, MC (February 2011). "A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: what have we learned in the last decade?". CNS Neuroscience & Therapeutics 17 (1): 4–31. doi:10.1111/j.1755-5949.2009.00116.x. PMID 20236142.
- ^ Debono MW, Le Guern J, Canton T, Doble A, Pradier L (April 1993). "Inhibition by riluzole of electrophysiological responses mediated by rat kainate and NMDA receptors expressed in Xenopus oocytes". Eur. J. Pharmacol. 235 (2-3): 283–9. doi:10.1016/0014-2999(93)90147-a. PMID 7685290.
- ^ Wokke, J (21 September 1996). "Riluzole.". Lancet 348 (9030): 795–9.doi:10.1016/S0140-6736(96)03181-9. PMID 8813989.
- ^ Kretschmer BD, Kratzer U, Schmidt WJ (August 1998). "Riluzole, a glutamate release inhibitor, and motor behavior". Naunyn Schmiedebergs Arch. Pharmacol. 358 (2): 181–90.doi:10.1007/pl00005241. PMID 9750003.
- ^ Azbill, RD; Mu, X; Springer, JE (July 2000). "Riluzole increases high-affinity glutamate uptake in rat spinal cord synaptosomes". Brain Res. 871 (2): 175–80.doi:10.1016/S0006-8993(00)02430-6. PMID 10899284.
- ^ Dunlop, J; Beal McIlvain, H; She, Y; Howland, DS (1 March 2003). "Impaired spinal cord glutamate transport capacity and reduced sensitivity to riluzole in a transgenic superoxide dismutase mutant rat model of amyotrophic lateral sclerosis". J Neurosci. 23 (5): 1688–96. PMID 12629173.
- ^ Wang, S.-J (January 2004). "Mechanisms underlying the riluzole inhibition of glutamate release from rat cerebral cortex nerve terminals (synaptosomes)". Neuroscience 125 (1): 191–201. doi:10.1016/j.neuroscience.2004.01.019.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.
P.S. : The views expressed are my personal and in no-way suggest the views of the professional body or the company that I represent.

 COCK SAYS MOM CAN TEACH YOU NMR
COCK SAYS MOM CAN TEACH YOU NMR


![[1860-5397-7-57-i20]](http://beilstein-journals.org/bjoc/content/inline/1860-5397-7-57-i20.png?max-width=550&background=FFFFFF)
![[1860-5397-7-57-i21]](http://beilstein-journals.org/bjoc/content/inline/1860-5397-7-57-i21.png?max-width=550&background=FFFFFF)
![[1860-5397-7-57-i22]](http://beilstein-journals.org/bjoc/content/inline/1860-5397-7-57-i22.png?max-width=550&background=FFFFFF)
![[1860-5397-7-57-i23]](http://beilstein-journals.org/bjoc/content/inline/1860-5397-7-57-i23.png?max-width=550&background=FFFFFF)