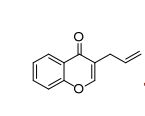
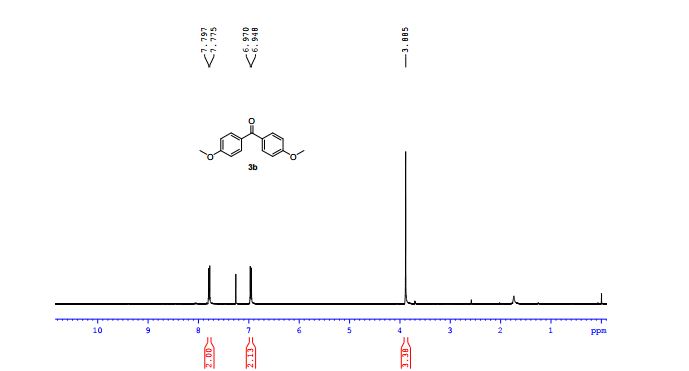
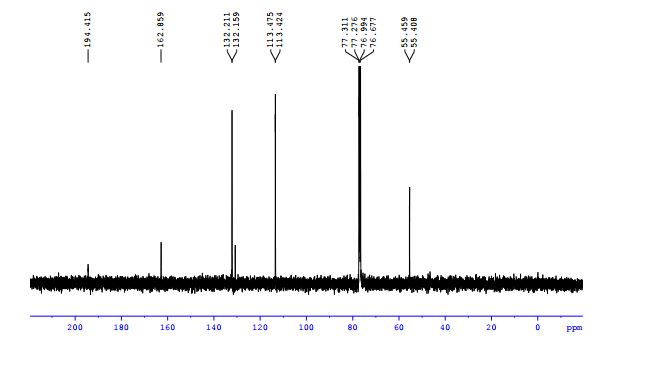
3-allyl-4H-chromen-4-one
5b (8.4 g, 45 mmol, 91% yield) as a yellow oil. GC (retention time): 3.571 min
HRMS (EI): m/z calcd for [C12H10O2]: 186,0681 (M+ ); found 186.0657.
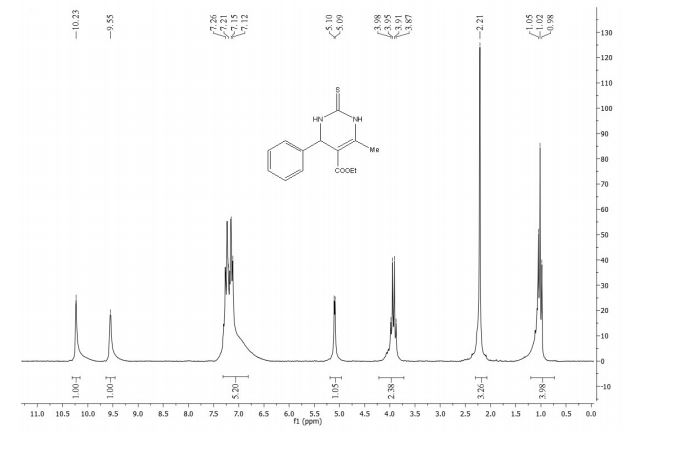
1H-NMR (400 MHz, CDCl3 ): δ/ppm = 8.18 (dd, J=8.0, 1.7 Hz, 1H), 7.70 (t, J=1.0, 1H), 7.58 (ddd, J=8.6, 7.1, 1.7 Hz, 1H), 7.43 – 7.28 (m, 2H), 5.92 (ddt, J=16.7, 10.1, 6.7 Hz, 1H), 5.22 – 5.04 (m, 2H), 3.20 (dt, J=6.7, 1.3, 2H).
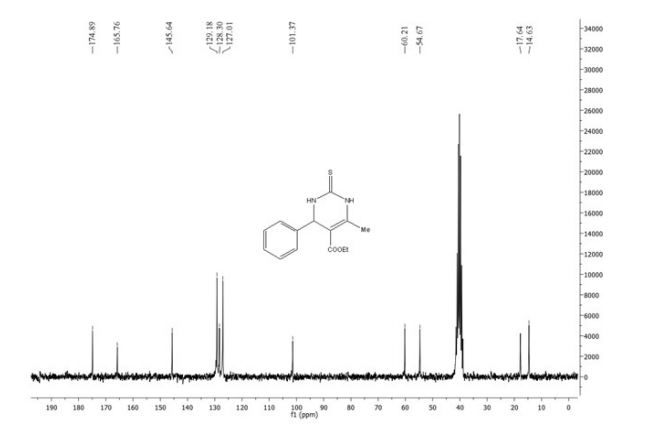
13C NMR (75 MHz, CDCl3 ): δ/ppm = 177.5, 156.6, 152.7, 134.7, 133.5, 126.0, 125.0, 123.9, 123.1, 118.1, 117.2, 29.8.
IR (Diamond-ATR, neat): /cm-1 = 3077.00 (w), 3068.00 (w), 3017.00 (vw), 2970.00 (w), 2941.00 (vw), 2900.00 (w), 1632.00 (s), 1608.00 (s), 1573.00 (m), 1464.00 (s), 1429.00 (m), 1398.00 (s), 1353.00 (s), 1321.00 (m), 1297.00 (m), 1282.00 (m), 1264.00 (w), 1226.00 (w), 1210.00 (m), 1181.00 (m), 1156.00 (s), 1141.00 (s), 1111.00 (m), 1027.00 (w), 1005.00 (m), 961.00 (m), 924.00 (s), 909.00 (s), 896.00 (s), 868.00 (w), 846.00 (s), 802.00 (m), 769.00 (s), 756.00 (vs), 712.00 (m), 690.00 (s).
mp: 34.0 - 36.0 °C
Practical Large-Scale Regioselective Zincation of Chromone Using TMPZnCl·LiCl Triggered by the Presence or Absence of MgCl2
† Department Chemie, Ludwig-Maximilians-Universität, Butenandtstrasse. 5-13, 81377 München, Germany
‡ Research & Development, Crop Science, Bayer AG, Alfred-Nobel-Strasse 50, Building 6550, 2.08, 40789 Monheim am Rhein, Germany
Org. Process Res. Dev., Article ASAP
DOI: 10.1021/acs.oprd.7b00032

Chromones are efficiently zincated in position C(3) in THF by using the commercially available amide base TMPZnCl·LiCl (TMP = 2,2,6,6-tetramethylpiperidyl). Additionally, in the presence of a Lewis acid such as MgCl2, zincation using TMPZnCl·LiCl occurs at C(2). These metalation reactions are carried out on a 50 mmol scale, and the metalation selectivities have been compared with the corresponding small-scale reactions (2 mmol). The resulting zinc organometallics undergo smooth reactions with various electrophiles, for example, Pd-catalyzed cross-coupling reactions or Cu-catalyzed acylations or allylations.