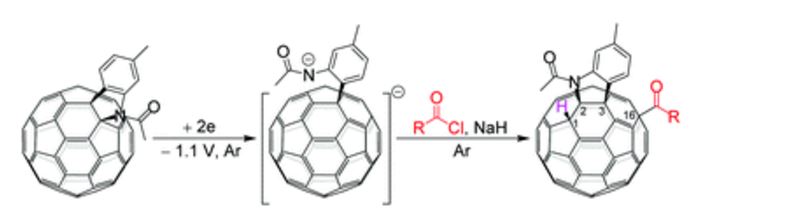
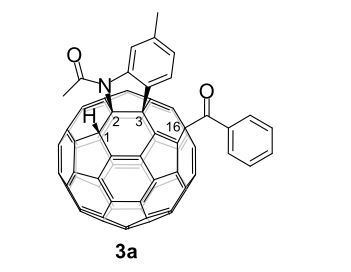
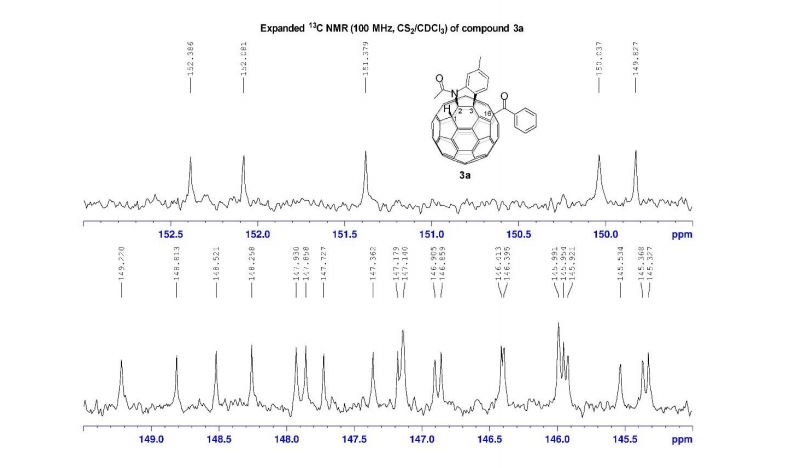
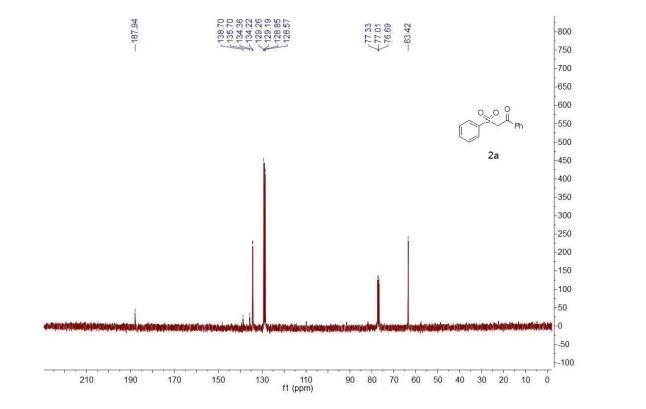
3a (11.2 mg, 38%) were obtained along with unreacted 1 (1.1 mg, 4%).
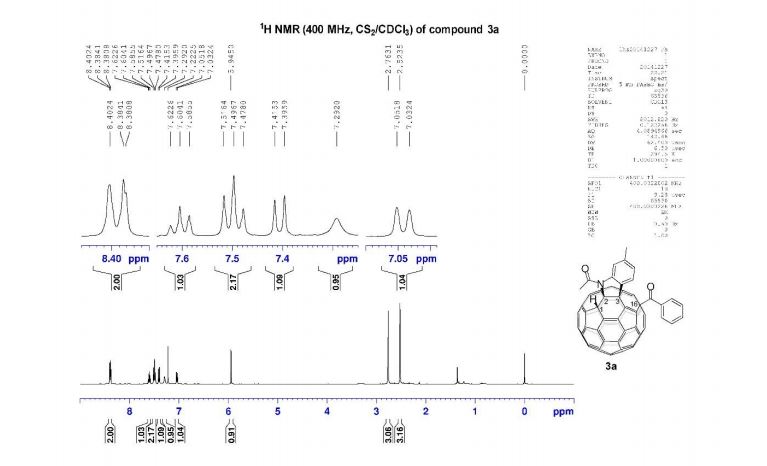
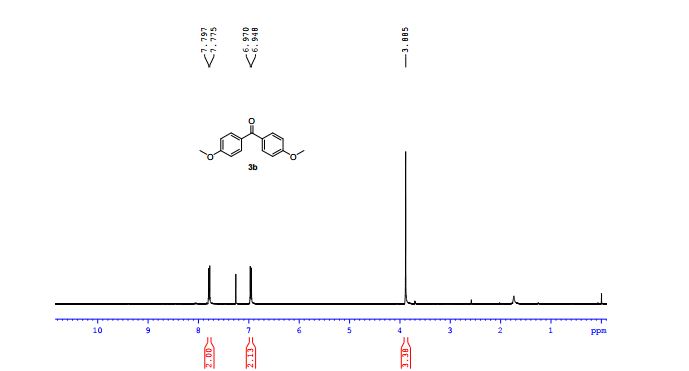
1H NMR (400 MHz, CS2/CDCl3) δ 8.39 (d, J = 8.0 Hz, 2H), 7.60 (t, J = 7.4 Hz, 1H), 7.50 (t, J = 7.7 Hz, 2H), 7.41 (d, J = 7.8 Hz, 1H), 7.29 (s, 1H), 7.04 (d, J = 7.8 Hz, 1H), 5.95 (s, 1H), 2.76 (s, 3H), 2.52 (s, 3H);
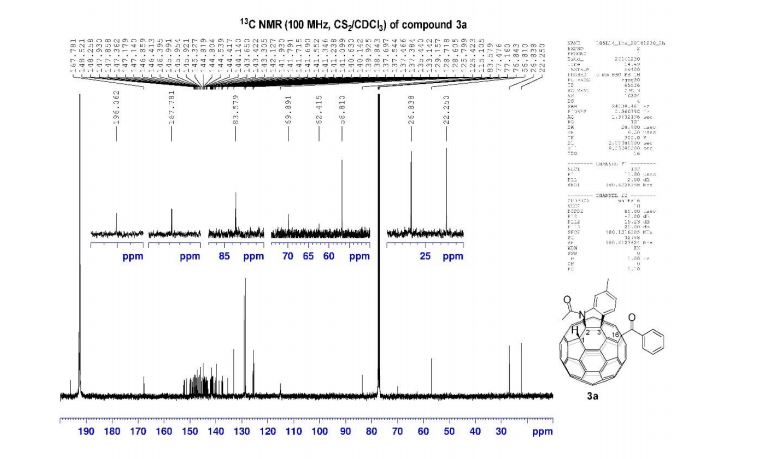
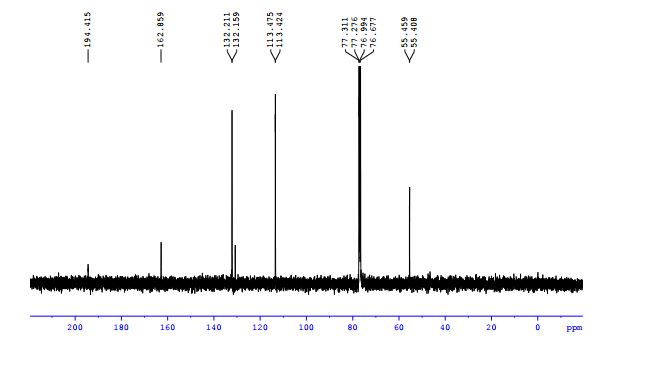
13C NMR (100 MHz, CS2/CDCl3, all 1C unless indicated) δ 196.06 (C=O), 167.78 (C=O), 152.39, 152.08, 151.38, 150.04, 149.83, 149.22, 148.81, 148.52, 148.26, 147.93, 147.86, 147.73, 147.36, 147.18, 147.14 (2C), 146.91, 146.86, 146.41, 146.40, 145.99 (2C), 145.95, 145.92, 145.53, 145.37, 145.33, 144.82 (2C), 144.80, 144.72, 144.54, 144.42, 144.31, 144.14, 143.84, 143.65, 143.42, 143.31, 143.05, 142.13, 141.93, 141.79, 141.72 (2C), 141.69, 141.55, 141.35, 141.24, 141.10, 140.63, 140.14, 139.93 (aryl C), 138.84, 137.70, 137.54 (aryl C), 137.47, 137.38, 135.44 (aryl C), 133.14 (aryl C), 129.16 (2C, aryl C), 128.72 (2C, aryl C), 128.61 (aryl C), 125.80 (aryl C), 125.42 (aryl C), 115.11 (aryl C), 83.58 (sp3 -C of C60), 69.89 (sp3 -C of C60), 62.42 (sp3 -C of C60), 56.81 (sp3 -C of C60), 26.84, 22.25;
UV-vis (CHCl3) λmax nm (log ε) 251.0 (5.1), 318.5 (4.6), 403.5 (4.0), 440.0 (3.9), 525.5 (3.2), 703.5 (2.5);
FT-IR ν/cm-1 (KBr) 2922, 2860, 1668, 1599, 1499, 1439, 1366, 1304, 1236, 1180, 1086, 1020, 964, 858, 802, 748, 691, 604, 528;
MALDI-TOF MS m/z calcd for C76H16NO2 [M+H]+ 974.1176, found 974.1165.
Regioselective acylation and carboxylation of [60]fulleroindoline via electrochemical synthesis
Abstract
A regioselective and highly efficient electrochemical method for direct acylation and carboxylation of a [60]fulleroindoline has been developed. By using inexpensive and readily available acyl chlorides and chloroformates, both keto and ester groups can be easily attached onto the fullerene skeleton to afford 1,2,3,16-functionalized [60]fullerene derivatives regioselectively. In addition, a plausible mechanism for the formation of fullerenyl ketones and esters is proposed, and their further transformations under basic and acidic conditions have been investigated.